
Karl Collins
Karl was born in the depths of Yorkshire, but moved to Manchester to study chemistry.
Following his Master’s in chemistry, which included a year working in pharma, he stayed on at the University of Manchester for PhD in organic synthesis with David Procter. While his chemistry improved his accent didn’t, and the mixture of northern influences were further aggravated when he moved to Germany for a postdoc in catalysis with Frank Glorius. Now speaking an awkward mix of northern English with German grammar and bad German with terrible grammar, Karl decided to remain in Germany and currently works as a medicinal chemistry laboratory head at Bayer Pharma, Wuppertal. He has dedicated interests beyond medicinal chemistry in photochemistry, contemporary catalysis and screening methodologies for reaction evaluation and exploration.
 Opinion
OpinionFully automated synthesis of fluorine-18 PET tracers
Making radioisotopes suitable for positron emission tomography
 Opinion
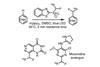
OpinionTaking benzyl fluorides beyond game changing reports
Decarboxylative cross-coupling to produce benzyl fluorides
 Opinion
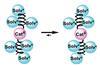
OpinionExploring nickel reactivity in C–H activation chemistry
Coming closer to an alternative for palladium
 Opinion
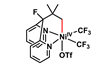
OpinionStereoselectivity with a twist
Helical ligands amplify solvent chirality to control reaction outcomes
 Opinion
Opinion‘Coffee machine’ synthesiser’s first steps
Cartridge-based machine enables non-experts to make heterocycles
 Opinion
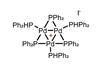
OpinionIn search of ultimate selectivity
A catalyst that reacts only with aryl iodides, spurning bromides and chlorides
 Opinion
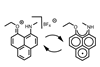
OpinionCombining Lewis acid and redox catalysis
Organic phenalenyl cations perform a dual role without transition metals
 Opinion
OpinionMaking light work of synthesis
The LED zeppelin is flying high, but is it all hot air asks Karl Collins
 Opinion
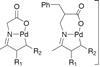
OpinionKetones perform at the palladium
C–H activation takes the stress out of organometallic couplings
 Opinion
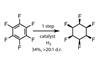
OpinionForcing fluorines into shape
Sometimes unnatural molecules can be more challenging to synthesise than natural metabolites
 Opinion
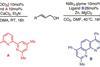
OpinionNickel catalyst couples alcohols and carbon dioxide
Flexibility and low cost make nickel a firm favourite in catalysis
 Opinion
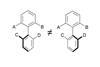
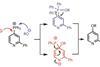
OpinionFunctionalising pyridines with phosphonium salts
Newly-independent research groups often bring new perspectives to synthesis
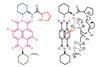
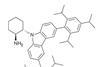 Opinion
OpinionLighting up crowded corners
Combining photocatalysis with organocatalysis opens doors to chiral quaternary centres
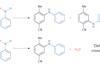 Opinion
OpinionAn odd couple
Coupling unactivated phenols with amines requires an unusual approach, as Karl Collins discovers
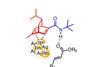 Opinion
OpinionScratching chiral surfaces
Heterogeneous asymmetric catalysis beyond hydrogenation is tricky, says Karl Collins
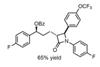 Opinion
OpinionA witches’ brew for trifluoromethylation
Karl Collins is surprised by the difficulty of a seemingly simple alkylation
 Opinion
OpinionDispelling nickel’s catalytic demons
Stable Ni(IV) complexes could help nickel rival its flashier platinum-group cousins, says Karl Collins