Gilead and its partners’ efforts to distribute generic lenacapavir could be derailed by cuts in international aid budgets
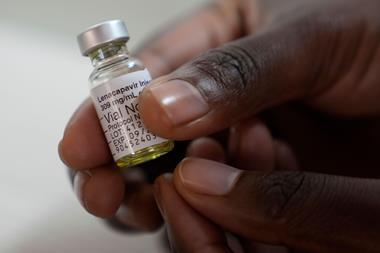
US approval of Gilead’s six-month HIV preventive injection, Yeztugo (lenacapavir), marks a significant advance in the global struggle against HIV–Aids. Two injections a year to provide near-total protection from infection is a huge step up in terms of both effectiveness and ease-of-administration, compared to daily tablet forms of pre-exposure prophylaxis (PrEP).
Assuming European and other approvals of the drug for PrEP follow relatively swiftly (it already has several approvals for controlling HIV in some groups of infected patients), expect at-risk populations in higher-income countries to quickly avail themselves of Yeztugo’s greater convenience and protection. Eventual annual sales are predicted to be at least £3–5 billion.

But the vast majority of people at highest risk of HIV infection live in lower and middle-income countries of sub-Saharan Africa and Central and South America. As detailed in this extended report in Science, Gilead and its partners invested significant efforts to ensure that diverse and critical segments of these populations were represented in clinical trials – including adolescents and pregnant women – to allow approval criteria to include them from the start. Since lenacapavir’s 2022 approvals for treating existing infections, the company has also already agreed licensing deals with six generics manufacturers to allow the drug to be distributed in 120 low- and lower-middle income countries. Gilead is supplying its own branded lenacapavir at no profit to 18 countries with high HIV burdens until its licensed generics manufacturers can meet demand.
Price negotiations for drugs are generally kept very private, but depending on the scale of manufacturing, academic estimates suggest generic lenacapavir could be supplied for around £20–40 per year, including a modest profit. Even at generic prices, however, charitable distribution programmes incur costs, and generally depend on foreign aid funding. With the US withdrawing from the World Health Organization and cutting USAID, which was the biggest funder of HIV programmes in the developing world, along with other major contributors like the UK making cuts to their foreign aid budgets, that funding is under threat.
There are also countries not covered by Gilead’s generics agreements, where infection rates are still high – including Brazil, Argentina and parts of eastern Europe. The company says it is pursuing accelerated regulatory registrations in countries with high infection rates, and will develop tiered pricing models and partnerships to enable wide access to the drug. Ensuring Yeztugo can fulfil Gilead chief executive Daniel O’Day’s ambition to ‘help end the HIV epidemic’ will still take significant effort and commitment both from within the company and outside.













No comments yet