Non-classical covalent pyramidanes surprise with high bonding ionicity
Polyhedra have fascinated chemists for decades. Now, an international team has indulged this passion and constructed a covalently bonded pyramidal structure in which the apex is a germanium, tin or lead atom and the corners of the square base a carbon, silicon or germanium atom each with attendant trialkylsilyl groups. Surprisingly, the team found that the bonding between the pyramidane compounds’ base and apex had a surprisingly strong ionic character.
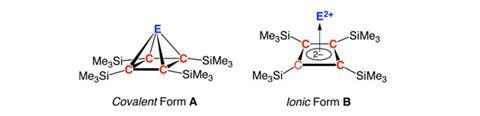
The methodology for the preparation of molecular pyramidanes has remained elusive. Semi-empirical studies in the late 1970s first hinted that pyramidanes might be stable. Subsequent theoretical analyses of greater and greater computational power ultimately led to the suggestion that they might have an experimentally achievable relative energy minimum. The logical conclusion building on those foundations was that pyramidanes might be synthetically accessible and isolable, astonishingly, say the researchers, at room temperature.
Although some square-pyramidal structures are known in organometallic chemistry, such as alkali metal salts or transition metal complexes of cyclobutadiene, no complexes of p-block elements, actual pyramidanes, in other words, have been reported so far. Given this earlier theoretical work and the potential for pyramidanes to bridge the organometallic and organic realms, they have been important targets for synthesis in this community. Now, a team led by Vladimir Lee and Akira Sekiguchi both of the University of Tsukuba, Japan, and Vladimir Minkin of the Southern Federal University, Rostov on Don in Russia, have created several.
The team reasoned that the group 14 elements might be the right region of the periodic table from which to fish a covalently bonded pyramidane. The researchers considered various strained cyclic compounds as their starting point and quickly homed in on a bicyclic carbene – bicyclo[2.1.0]pent-2-en-5-ylidene – as the most suitable precursor, with its fairly low isomerisation barrier to the target pyramidane. They worked from their recently synthesised cyclobutadiene dianion alkali-metal salt and reacted it with readily available dioxane complexes of dichlorogermylene or dichlorostannylene to generate the bicyclo[2.1.0]pent-2-en-5-ylidene and thence the various group 14 element pyramidanes.
They used x-ray diffraction, NMR and Mössbauer spectroscopy to verify their findings experimentally and high level computational studies to lend support to the bonding within pyramidanes. They point out that while the compounds are indeed covalent, there is an ‘extraordinarily high degree of ionicity’ in the pyramidal structure between apex and base bonds in these non-classical covalent compounds.
‘We will next focus on the study of pyramidanes reactivity: by taking advantage of the intrinsically weak apex-to-base pyramidal bonds, we intend to employ our pyramidanes as an indispensable source of the bare unsubstituted apical atom whose exceptional reactivity may lead to unprecedented compounds,’ Sekiguchi tells Chemistry World.
‘Pyramidanes have fascinated chemists for the last 50 years,’ says Yitzhak Apeloig of Technion-Israel Institute of Technology. ‘The question of whether they can be prepared, the bonding scheme that holds the atoms together in this particular geometry and so on has intrigued both theoreticians and experimentalists. Sekiguchi’s paper provides an important breakthrough on these fundamental questions.’
References
V Y Lee et al, Organometallics, 2016, 35, 346 (DOI: 10.1021/acs.organomet.5b00924)











No comments yet