Cobalt could cut costs for pharma ingredients
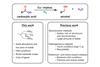
Carboxylic acids and esters hydrogenated without the need for expensive precious metals

Carboxylic acids and esters hydrogenated without the need for expensive precious metals