Rotaxane raises the bar for self-replicating chemical systems
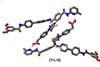
Self-replicating rotaxane may open the door for autonomous chemical synthesis

Self-replicating rotaxane may open the door for autonomous chemical synthesis