Can dye-sensitised solar cells compete with silicon and emerging alternatives? Phillip Broadwith investigates
In 1991, Michael Grätzel and Brian O’Regan from the Swiss Federal Polytechnic School in Lausanne (EPFL) published a landmark Nature paper. It described a dye-sensitised solar cell (DSSC) that could rival inorganic materials like silicon. Although the idea of a dye-sensitised cell had been around for decades, their poor performance had made them little more than an academic curiosity. Since then, DSSCs have been lauded as potentially cheap, flexible, colourful and customisable solar energy collectors, with Grätzel and O’Regan tipped as potential Nobel laureates.

False idols
However, as Gavin Tulloch from Australian DSSC company DyeSol explains, focusing on efficiency alone is missing the point. ‘A dye solar cell is quite different to silicon. They’re large energy generators rather than large peak power generators,’ he says.
That large energy generation comes from the fact that a DSSC produces more or less constant voltage all the time, Tulloch adds. DSSCs don’t need bright sun, in fact they work well in dappled or hazy light conditions, ‘the sort of light that exists where people actually live, not the sort of light that exists in deserts’, he says.
Unlike silicon cells, which are very sensitive to the angle of incoming light, dye cells work well with light coming in at different angles, such as might happen in urban environments. ‘Angles are wonderful for us,’ Tulloch points out, ‘if you can get a photon through the outside transparent layer, then the higher the angle the better, because it has a better chance of hitting a dye molecule. We even put reflecting particles into our cells to bounce the light around and trap photons into the dye.’
In 2009, DyeSol ran an experiment at their Australian headquarters in Queanbeyan to compare the performance of their DSSCs against silicon and CIGS panels oriented vertically as if they were on the sunny north facade of a building (equivalent to a south-facing facade in the northern hemisphere), and showed that the DSSC produced around 30% more power than the silicon and 49% more than CIGS on a sunny day, with even bigger differences on cloudy or hazy days.
However, to Tulloch’s frustration, convincing investors is a different matter. ‘They say: “Oh, but your efficiency is only 5%,” or 8% or whatever it happens to be. It’s difficult to get across that total energy produced is greater. They don’t want to believe that, because they’ve been taught for the last 20 years that the only thing that matters is efficiency – artificial efficiency, measured in an environment that I like to say occurs for one day of the year in the spring at the top of the Eiger.’
Buildings as power plants
DyeSol has worked to engineer cells that can be scaled up to modules that will last for 25–50 years outdoors in northern Europe. To do this, they have relied on tweaking the robust iodide–triiodide electrolytes and ruthenium dyes that have been the mainstay of DSSC technology for years. While this may not give them record-breaking efficiency, it does make for reproducible and robust cells.
In collaboration with Tata Steel, the company is developing building-integrated photovoltaic products, such as sheet steel cladding for industrial buildings that incorporates DSSC modules. ‘They realised that what they had to do was develop a building product that just happened to produce power, rather than a solar cell that you put on a roof,’ says Tulloch. ‘We’re talking about integrating them into bus shelters in north Wales, where nothing else would work because there’s not enough sun and it’s generally wet; or for portable toilets after natural disasters, which don’t normally have lights in them. Tata is mainly interested in what they call “large sheds” – warehouses and industrial buildings of around 10,000–100,000m2 – and that’s a mini power station.’
Division of labour
Nevertheless, improving efficiency would push energy generation even higher. So why has efficiency not increased for DSSCs as fast as it has for other technologies? Henry Snaith, who develops dye cells at the University of Oxford, UK, explains that the great advantage of DSSCs – which is also a challenge – is the fact that each component is separate in its functionality, which in principle means that they can be independently optimised for their function (see ‘anatomy of a DSSC’ below for an explanation of the components of a typical DSSC and their functions). ‘What you don’t want is a single material or single component playing lots of roles,’ says Snaith, ‘because it will be non-ideally optimised to give the best net effect, but not necessarily ideal for any of its individual roles.’ However, the flip side of this is that researchers need to understand exactly what each component is doing. And because the components are not working in isolation, changing one thing can mean you need to rejig the rest of the cell to get the full benefit.
For example, he adds, in solid state DSSCs, a lithium salt is often added to the hole transporter because it increases efficiency. ‘But it actually does two roles, and it’s optimised for one at a much lower concentration than the other.’ This means that in the fully optimised solar cell, which is optimised for overall efficiency, neither function is perfect. ‘If you could separate those two functions with two separate components, you should be able to get better, more efficient solar cells,’ Snaith concludes.
But as Nicolas Tétreault, who works with Grätzel at EPFL points out, in the last couple of years, developments in both light-harvesting dyes and the dye-regenerating electrolytes have begun to push efficiency levels up again. ‘There has been a big shift away from ruthenium organometallic dyes towards all-organic sensitisers,’ he says. He also points to people using combinations of dyes that absorb in different parts of the spectrum, which means the cell can generate a higher current as absorbing more photons means stimulating more electrons. ‘In our last cell there were two dyes,’ he says, ‘one completely organic and one based on a zinc porphyrin.’
Another area that the EPFL group, among others, is working on is replacing the iodide–triiodide electrolyte found in most DSSCs. This mixture is highly corrosive, so eliminating it has lots of advantages in terms of cell engineering and lifespan. ‘But there is also a big loss of energy when an electron goes from the electrolyte to reduce and regenerate the oxidised dye,’ says Tétreault. ‘We were losing around 600mV.’ Switching to a cobalt-based electrolyte can reduce this energy loss, allowing the cell to generate a higher voltage and hence more power.
The key thing is the combination, as Tétreault explains. ‘The cobalt electrolyte is a bulky molecule, so it diffuses through the pores in the TiO2 layer quite slowly. But if you have a dye that can harvest more light, you can make the cell thinner. Then you can get away with an electrolyte with slower diffusion, and you end up with cells that use half as much material but have the same or higher efficiency. Using both the new dye and electrolyte, we’ve been able to reach 13% efficiency [at 0.5 sun],’ he adds.
Solar without the sun
However, these new dyes and electrolytes do have some disadvantages – they tend not to stand up to the demands of outdoor solar power for long enough to be commercially viable. ‘Outdoors you’ve got extreme temperatures, along with variable and rapidly changing light levels, which leads to rapidly changing temperatures as well,’ says Mark Spratt from Welsh DSSC manufacturers G24i. ‘Indoors you don’t have that.’

‘Our cells work very well at low light levels,’ he says. ‘Fluorescent lighting is particularly efficient because the light it puts out is predominantly in the visible spectrum, and the dye that we use has a close spectral response to the human eye, so if a light source is good for human vision, it’s good for the DSSC as well.’

Wireless keyboards, TV remote controls, security sensors and e-readers like Amazon’s Kindle that use low-voltage e-paper displays are all candidates for light-harvesting power. ‘Removing the need for batteries or external chargers frees up designers to play around with the form of these devices,’ adds Spratt.
And when the MGM Grand hotel in Las Vegas, US, wanted to replace the curtains in its rooms with remote-controlled electric blinds, it was G24i that provided the solution. ‘Installing traditional motorised blinds would have involved chasing wires through the walls, which would have cost about $4.5 million dollars over 5000 rooms,’ says Spratt. ‘So a DSSC-powered system that charges a battery within the blind was a very large cost saving for them. They used our technology because the windows are heavily tinted, so the light levels are much lower than outside.’
Clearing roadblocks
But for O’Regan, now at Imperial College London, UK, the challenge is not to try and eke out a few more percentage points of performance by designing new cells, but to get to grips with some of the fundamental processes going on within the cells, which might throw up serious roadblocks in future development. Despite all the work that has been done on DSSCs over the last 20 years, he says, there are still several fundamental questions about how the cells work that we’re only just beginning to investigate.
This is another reason to get rid of the fixation on efficiency alone. ‘We do not make enough measurements,’ he says. ‘Very few cells are given more than a cursory “this is the efficiency at 1 sun”.’ This risks missing performance advantages under different light conditions, or prematurely dismissing ideas that could solve other problems that don’t show up from efficiency measurements. It also gives very little insight into what processes are holding back efforts to improve performance. ‘It’s all about measuring a wider spectrum of information,’ he adds.
One of the possible roadblock problems is electron injection – when the excited dye molecule, having absorbed a photon, transfers one of its electrons to the TiO2 layer. ‘No one has seriously attempted to evaluate injection, because everyone has gone with the flow that they’ve found femtosecond injection and assumed it was all like that, so there was no need. What we’ve discovered is that there’s a large heterogeneity of rate constants,’ says O’Regan. ‘People don’t like thinking about heterogeneity. They like to give a single time constant.’
O’Regan’s group has now taken measurements that indicate there are significant fractions of injection occurring at all timescales from around 1.5ns to a few femtoseconds. ‘And that’s really bad,’ he says, ‘because when you try to extract more energy from something, you always slow the rates down. If you had a cell with only femtosecond injection, you would immediately start to change the materials to extract more energy, in which case you slow it down a bit, but that’s OK. But you can’t do that if you also have injection in the nanosecond time, because if you slow that down to microsecond time, you lose it completely.’
One way to explain the heterogeneity of injection rates, says O’Regan, is in terms of the distance the electron has to hop into the TiO2. ‘If one dye molecule is bonded to the TiO2 in one way and another one is bonded in another way, or it’s bonded to a tiny piece of silica that’s somehow got onto the surface, it will be further away, and so injection will be slower.’ However, he is quick to point out that there are several other potential explanations for the variation, and it is by no means a solved problem. The key thing is how it relates to cell performance. ‘What I suspect is that this could be partly responsible for the difference between really good and not-so-good cells,’ he says, referring to the fact that there can be a lot of variability in performance – even across different cells made exactly the same way. ‘Even the best groups have this variation in output,’ he adds.
Looking beyond
But while O’Regan agrees that progress on increasing efficiency has not been fast enough to date, he is also convinced it is possible to make more efficient dye cells, even if there are some fundamental roadblocks to overcome. ‘It all hinges on getting a working infra-red dye and an electrolyte for that dye,’ he says. ‘But because everyone thought injection was very fast, people haven’t paid much attention to the luminescence lifetime of the dyes they were making. If you want to extract the maximum energy from any system, it has to have a long lifetime.’ He explains that there are infra-red dyes with reasonable lifetimes, ‘but they aren’t really being pushed in that direction, except by people in other fields – in medical imaging, people are looking at dyes with high luminescence efficiency, and luckily for us, that’s exactly the same thing as long lifetime.’
Whether or not the dye solar road will lead to Stockholm remains to be seen, but it is clear that DSSCs are beginning to find their feet alongside their silicon cousins. The next few years will be exciting for both academic and industrial players, as G24i’s Spratt points out: ‘We’re on the cusp right now – we’re really starting to get there.’
Phillip Broadwith
Anatomy of a DSSC
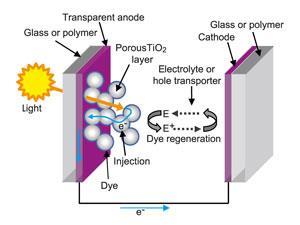
When the dye is excited by absorbing a photon, it injects an electron into the TiO2 layer, where it is conducted away. To prevent the oxidised dye from decomposing, it needs to be regenerated by replacing its lost electron. This electron is delivered by the third principle component of the cell: the electrolyte. In many cells this is a liquid solution, but because of the potential problems of leaking, efforts are being made to find solid-state equivalents. These are known as hole-transport materials, as the electron used to regenerate the dye leaves a positively charged ‘hole’ in the material, which hops its way from molecule to molecule back to the opposite electrode to be refilled with an electron. The majority of DSSCs use an electrolyte based on the iodide–triiodide (I––I3–) redox couple.












No comments yet