Electrical conductors can be strongly and reversibly adhered to soft matter such as gels or plant and animal tissue when a small electric field is applied. The surprising finding could find use in biomedical, materials or robotic applications. ‘I feel like I have found a secret in nature,’ says Srinivasa Raghavan, who led the research.
Typically, adhesion between soft gel-like substances and hard solids is possible only with materials that have been modified in some way, such as with azides and alkynes – to be linked through cycloaddition reactions – or with catechol groups to mimic how mussels stick to rocky surfaces. Previous work has shown that cationic and anionic gels can adhere through the application of a small external electric field and the Raghavan group has proved that such electroadhesion works between a gel and naturally anionic animal tissue.
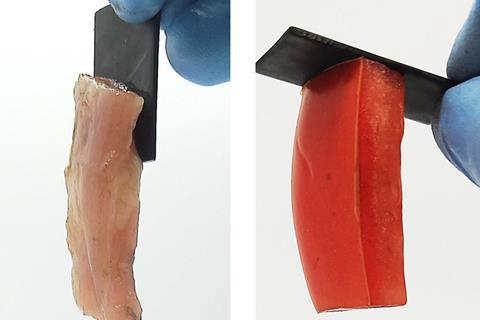
Now, Raghavan and his PhD students Wenhao Xu and Faraz Burni have discovered that hard, conductive materials such as graphite, tin, copper and lead can be reversibly electroadhered to a range of soft, water-containing substances. By applying a low DC charge across the two surfaces for a short period, these soft and hard materials become bonded with a strength that exceeds 150kPa and is an order of magnitude higher than that previously seen between two gels.
The team found that hard−soft electroadhesion could occur with anionic, cationic and nonionic gels, including fruit and animal tissue containing sufficient salt content, but that some anionic gels adhere only at the anode, and others only at the cathode. Using an acrylamide hydrogel to screen conductive metals, they found that hard−soft electroadhesion occurred only at the anode and correlated with the electrochemical series. Relatively inert metals with positive reduction potentials, such as tin and copper, adhered to the gel, whereas more reactive metals with negative reduction potentials, such as titanium and iron, failed to adhere. The researchers hypothesise that the less reactive metals allow the polymer chains of the gel to oxidise and establish chemical bonds with the metal surface. When the polarity of the electric field is reversed, the polymer is reduced and bonding ends.
Comparison of acrylamide gel slices taken from near the anode shows evidence that an electrochemical reaction has consumed the amide group and generated new difficult-to-identify bonds. As hard−soft electroadhesion is possible with a wide range of conductors and gel-like materials, the precise nature of the electrochemical changes will differ based on the chemistries of the constituents. ‘We’ve shed some light on the mechanism using spectroscopic techniques, but we are nowhere near completely understanding what’s going on,’ says Raghavan. ‘I don’t understand why in some cases it doesn’t work, but in a variety of cases it does work. That’s what makes this entire phenomenon unique and different and unexpected.’
To demonstrate potential applications, the research team used hard−soft electroadhesion to construct a battery with a hydrogel electrolyte component, electrogrippers capable of picking up and dropping off gels, and load-bearing combinations of hard and soft materials. These experiments serve as proof-of-concept demonstrations, but the team is as yet unsure of where their new technique may find use. ‘We have a novel phenomenon. We have some novel material properties, but where it would go application-wise remains to be seen,’ says Raghavan. ‘The story is yet unfinished in that regard.’
Kevin Turner, who is a professor of engineering and applied mechanics at the University of Pennsylvania, US, finds the research fascinating. ‘It’s a very surprising result, not just that you can form the bond, but that it is electrochemical in nature and it can be reversed.’ He believes that hard−soft electroadhesion’s functionality within water will lead to useful applications, potentially within medicine and healthcare. ‘One of the unique attributes of this technique is the ability to form bonds in aqueous environments because it’s just hard to do this with many other approaches,’ he explains. Turner is also intrigued by potential applications in gripping – ‘if there’s a way to enhance the speed, I think it could open up many opportunities in pick-and-place type processed and robotic applications’.
Raghavan plans to continue trying to understand the applications for, and mechanisms behind, hard−soft electroadhesion, but first needs funding. ‘This was all work done on the side. We never got any funding for it. So, I think that is the place I need to start.’
References
W Xu, FA Burni and SR Raghavan, ACS Cent. Sci., 2024, 10, 695 (DOI: 10.1021/acscentsci.3c01593)














1 Reader's comment