A new environmentally-friendly technique that uses arrays of strings to extract lithium from brine, and potentially even seawater, could help meet growing global demand for the metal, which is needed for electric vehicle batteries and grid storage as the world moves towards electrification and zero-carbon energy.
At present, lithium mining requires a lot of energy, chemicals, land and time. It’s also limited to only a few parts of the world where lithium occurs in high enough concentrations and where conditions are right for evaporation ponds, including Australia and South America. The most common mining method involves pumping brine from underground aquifers to form vast pools on the surface. Millions of litres of water are then left to evaporate for up to 18 months, ultimately allowing lithium carbonate to form from the lithium chloride salts. However, such operations are also known to contaminate groundwater, and harm ecosystems and indigenous communities.
Now, Z Jason Ren’s group at Princeton University, US, has devised an efficient and self-concentrating crystallisation technique that selectively harvests lithium from both brine and seawater by dangling the ends of specially designed strings into the liquid. ‘We do not need to apply additional chemicals, as is the case with many other extraction technologies, and the process saves a lot of water compared to traditional evaporation approaches,’ explains Ren.
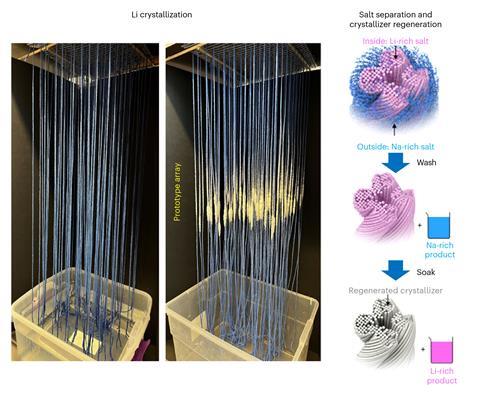
The technique uses engineered cellulose fibres, which the team optimised for capillary action and evaporation. At their core, the fibres are water loving, while their surface is water repellant. Fibres were first spun into a tight thread. Four of these threads were then twisted together to create strings, which experiments showed could draw water higher up.
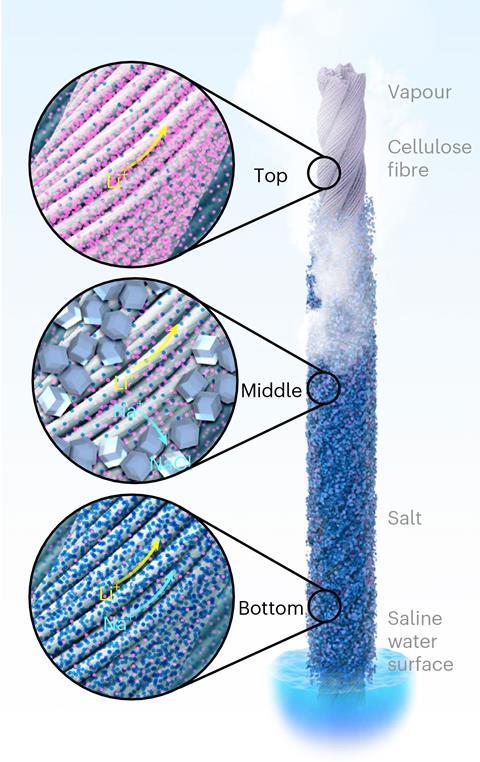
When the strings were dipped in lithium-containing salt water, the researchers observed the strings draw up the liquid through capillary action, just as plants suck water up from their roots to their leaves. The water then began to evaporate from the strings’ surface to leave behind lithium and sodium ions. As more brine was drawn up and evaporated, the salts concentrated to form sodium chloride and lithium chloride crystals.
Importantly, the crystallisation of sodium and lithium salts occurs at separate points on the string due to their different solubilities, meaning the lithium chloride could be harvested easily without additional processes. The team created a 100-string array showing how it can be scaled up.
‘Our process is like putting an evaporation pond on a string, allowing us to obtain lithium harvests with a significantly reduced spatial footprint and with more precise control of the process,’ says, study co-author Sunxiang (Sean) Zheng, who is leading the launch of a startup, PureLi, to develop the technology.
‘The authors’ ingenuity is about applying a technique that is known to work very well for other brines to this much more valuable brine,’ comments Shihong Lin, an environmental engineer at Vanderbilt University, US. ‘Using this low-cost and chemical-free approach can easily increase the productivity of current brine lakes by many-folds and potentially quench our thirst for lithium.’
However, Lin would like to see more experiments that use real salt-lake brines that contain components that could cause challenges. ‘For example, magnesium chloride has a high solubility and will likely end up in the lithium-rich region [of the strings], requiring subsequent separation like what is currently practiced,’ he explains. ‘Another concern is the presence of barely soluble minerals that may form scale on the strings and undermine the reusability of the system.’
References
X Chen et al, Nat. Water, 2023, 1, 808 (DOI: 10.1038/s44221-023-00131-3)











No comments yet