Molten salts put carbon dioxide to work
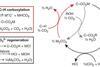
Scientists discover a simple way to form carbon–carbon bonds from carbon dioxide without using synthetic or biological catalysts

Scientists discover a simple way to form carbon–carbon bonds from carbon dioxide without using synthetic or biological catalysts