Sugar solution to toxic gold recovery
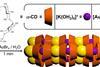
The environmental legacy of salvaging gold from electronic waste can be dramatically cut using corn starch instead of cyanide

The environmental legacy of salvaging gold from electronic waste can be dramatically cut using corn starch instead of cyanide