
Emily Cuffin-Munday
Emily completed a master’s degree in chemistry at the University of Manchester in 2021. Her research project focused on a computational investigation of the structures and photophysical properties of chiral graphene nanoribbons and exploring possible bottom-up syntheses for these materials. She joined the Royal Society of Chemistry as a publishing editor in 2022 and moved to a development editor role in 2023.
Outside of work, Emily enjoys running, hiking and climbing, as well as the odd crochet project!
 Research
ResearchUnexpected stability theorised in positron-bound beryllium dimers
Simulations challenge conventional ideas about positronic interactions
 Research
ResearchCarbon nanotubes made from waste carbon dioxide produce surprising plasma when microwaved
Carbonate electrolysis seems to incorporate transition metals into carbon nanotubes, which sees them absorb microwave energy more efficiently
 Research
ResearchQuantum tunnelling explains why several supposedly stable benzene isomers will never be made
Research highlights importance of considering quantum effects in computational studies on strained molecules
 Research
ResearchBad habits obscuring thermodynamic reality of photocatalytic reactions
Dubious assumptions and contentious nomenclature muddying the literature
 Research
ResearchOrganocatalyst deconstructs mixed plastic waste into monomers
Step towards closed-loop recycling
 Research
ResearchClick chemistry with sound-induced mechanocatalysis
Researcher behind work says they ‘could be very close to a perfect way to conduct green chemistry’
 Research
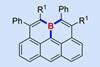
ResearchBoron-doped olympicenes are surprisingly stable
By possessing useful electronic properties, boraolympicenes could have potential applications in organic electronics
 Research
ResearchSurprise as electric fields found to cleave bond homolytically
Study also uncovers that the reaction rate in a field increases linearly with the solvent dielectric constant
 Research
ResearchGigantic database of building blocks will help artificial intelligence uncover new organocatalysts
Publicly available dataset containing thousands of structures could help chemists develop data-driven reaction optimisation methods for organic synthesis
 Research
ResearchComputational study says polonium can form hydrogen bonds
Bonds driven by relativistic effects, rather than electronegativity differences