Researchers in China have synthesised planar boron-doped olympicenes via a simple one-pot reaction. These boraolympicene compounds exhibit surprising chemical stability and electronic properties that could see them find use within organic electronics.
Polyaromatic hydrocarbons (PAHs) have extended π-networks that are responsible for their unique physiochemical properties. Olympicene, a PAH whose shape resembles the Olympic rings, was first synthesised and imaged in 2012. But real-life applications of olympicene and its radicals have been limited because they need bulky protecting groups to stabilise them.
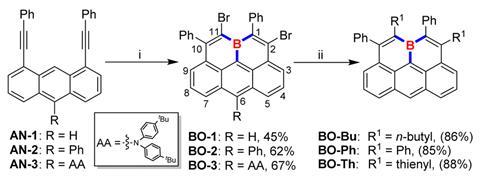
Heteroatom doping is a common strategy for controlling the physiochemical properties of PAHs. In particular, using three-coordinate boron atoms as dopants can create PAHs with low lying LUMOs, increased luminescence and strong Lewis acidity. Now, Kun Yang, Zebing Zeng and their colleagues at Hunan University have developed a one-pot triple borylation-based double-fold borocyclisation reaction for synthesising olympicenes featuring boron at the concave 11a-position. The boraolympicenes exhibited good chemical and thermal stability, without relying on bulky protecting groups. Because the synthesis is modular, the researchers could control the boraolympicenes’ physiochemical properties, giving them wide range absorbance and low-lying LUMOs. And varying the substituent size at the 6-position led to distinct molecular stacking behaviours between the molecules.








No comments yet