Researchers in India have calculated that polonium can form hydrogen bonds.1
Polonium is a naturally occurring radioactive element and its compounds engage in soil, marine and environmental chemistry. With Russia curtailing gas supplies to Europe, fracking for shale gas looks set to increase. Fracking could cause the release of polonium compounds into our atmosphere, such as Me2Po, which is produced by aerobic marine microorganisms. Increasing awareness of how such compounds could impact environmental and marine chemistry is therefore important.
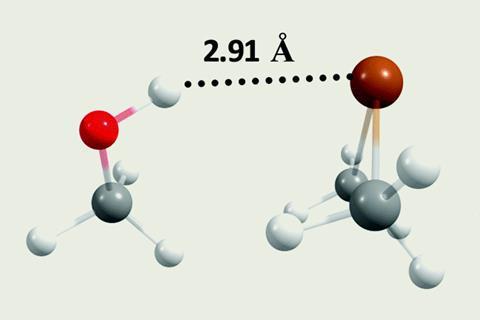
Hydrogen bonds are non-covalent interactions that are prevalent in biomolecular chemistry and understanding them can help explain complex biological processes and systems.2 Conventionally, hydrogen bonding is thought to be caused by a difference in electronegativities between the interacting atoms. However, in 2019, Himansu Biswal and his research group from the National Institute of Science Education and Research in India uncovered that the strength of X-H···S/Se hydrogen bonds was down to polarisability and dispersion.3
The most recent work from Himansu Biswal’s group began by questioning whether hydrogen bonds with polonium (X-H···Po) could exist. Using density functional theory (DFT), they have shown how X-H···Po hydrogen bonds can form, using Me2Po as the hydrogen bond donor. Strength-wise, the X-H···Po hydrogen bond is comparable to conventional hydrogen bonds (10–30kJmol-1). They also performed two-component relativistic DFT calculations and discovered that, unlike other chalcogen complexes, the relativistic effect influences hydrogen bonding in polonium complexes.1
References
1 K D Tulsiyan et al, Phys. Chem. Chem. Phys., 2022, 24, 17185 (DOI: 10.1039/d2cp01852g)
2 D K Sahoo et al, J. Phys. Chem. A, 2019, 123, 2227 (DOI: 10.1021/acs.jpca.8b12003)
3 A Chand et al, Acc. Chem. Res., 2020, 53, 1580 (DOI: 10.1021/acs.accounts.0c00289)








No comments yet