Researchers have developed a new way of performing direct mechanocatalysis.1 The method uses resonant acoustic mixing and offers a simple, scalable and solvent-less alternative to traditional copper-catalysed azide–alkyne click coupling (CuAAC). Tomislav Friščić, at the University of Birmingham, UK, who led the work, says it could change how chemists think about chemical synthesis by allowing them to ‘switch completely from solution-based chemistry to essentially solvent-less or solvent-limited mechanochemical environments’. ‘We could be very close to a perfect way to conduct green chemistry,’ Friščić adds.
Direct mechanocatalysis was first reported by James Mack and colleagues at the University of Cincinnati, US, in 2009.2 It uses the ball or vessel walls in a ball mill as metal catalysts. However, there are various drawbacks to current direct mechanocatalysis strategies. Tim Hanusa, a metal-based mechanochemistry expert from Vanderbilt University in the US, tells Chemistry World that ‘[the grinding media] are not always innocent players when it comes to mechanochemical reactions, as the metal in the balls can function as a reagent’ which can complicate syntheses.
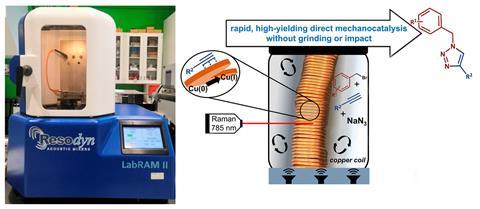
Now, Friščić and his colleagues have established a direct mechanocatalytic method that doesn’t require milling or crushing media. It uses a simple coil of copper wire to catalyse an azide–alkyne click coupling reaction under resonant acoustic mixing (Ram). A Ram machine vibrates the reaction vessel at a frequency of 60Hz so that the reaction mixture, in this case benzyl bromide and phenylacetylene, is gently shaken around the copper coil to catalyse the coupling reaction. The group from Birmingham tested their Ram mechanocatalysis technique to synthesise the anticonvulsant drug rufinamide. They scaled the synthesis up to the gram-scale and showed they could access this functional compound without the high temperatures or multistep processes currently used in solution.
The mechanism for direct mechanocatalysis has been subject to debate. Some believe the catalysis happens at the metal surface of the balls and others think it occurs on abraded metal particles resulting from the impact of the milling.3 Friščić’s group monitored the progress of their click coupling in real-time using Raman spectroscopy and observed zero-order, linear kinetics. This is what you expect to see in a solution synthesis and gives a ‘really strong indication that this is surface reactivity’, says Friščić. They combined their Raman spectroscopy with NMR spectroscopy to demonstrate that the reaction wasn’t progressing through any of the leached copper. This Ram technique is a more primitive alternative to solution synthesis and ‘mechanistically you might not actually be changing anything, but the [synthetic] route is more efficient’, says Deborah Crawford, a mechanochemical synthesis expert from the University of Bradford, UK.
The only obvious limitation of this technique is the expense of the Ram machine but ‘once you buy [or have access to] the Ram, afterwards it’s actually quite cheap to keep going,’ Crawford says. Friščić is currently involved in setting up a mechanochemistry facility in Birmingham that aims to make mechanochemistry equipment accessible to more researchers.
Friščić’s group plans to scale up the direct mechanocatalysis technique they have developed to between one and 100 kilograms, and also explore other syntheses with it.
References
1 C B Lennox et al, Chem. Sci., 2023, DOI: 10.1039/d3sc01591b
2 D A Fulmer et al, Green Chem., 2009, 11, 1821 (DOI: 10.1039/b915669k)
3 S Hwang, S Grätza and L Borchardt, Chem. Commun., 2022, 58, 1661 (DOI: 10.1039/d1cc05697b)













No comments yet