
Water confined in nanoscale channels has dramatically different electrical properties from bulk water, researchers at the University of Manchester have demonstrated. The differences, which include up to 100,000-fold greater conductivity along the channel, are thought to arise from alterations to the hydrogen-bonded network in this quasi-2D environment. The findings could have a significant impact on scientists’ understanding of biochemical processes.
Water is the most ubiquitous liquid on Earth and also the most effective solvent. This is largely because its hydrogen bonding gives it a high polarisability, allowing it to screen Coulomb interactions between molecules. It also has an unexpectedly high electrical conductivity for a wide-bandgap insulator – something that is itself unusual as polarisability normally hinders conductivity. However, many of water’s key reactions – including solvation itself – do not take place in the bulk but at its interface with another material, and this has led to intense study on the 2D hydrogen bonding in interfacial water to better model such processes.
Researchers at the University of Manchester, led by microscopist Laura Fumagalli and 2D materials expert and graphene Nobel laureate Andre Geim, have extended this by pushing the water through channels so narrow that the interfacial effects begin to interact. ‘We study the single interface when the channel is relatively thick and then the double interface when they are squeezed very much,’ says Fumagalli. ‘That is what we call strong confinement.’
In 2018, the researchers filled nanometre-scale channels of graphite and hexagonal boron nitride with water. They then applied an AC electric field and used a form of scanning probe microscopy called scanning dielectric microscopy to analyse the field perpendicular to the channel ie directly under the tip. ‘Essentially, the water was electrically dead,’ says Fumagalli – a finding that was controversial. ‘The large dielectric constant [a measure of polarisability] was reduced to a minimum and we couldn’t see any conductivity.’
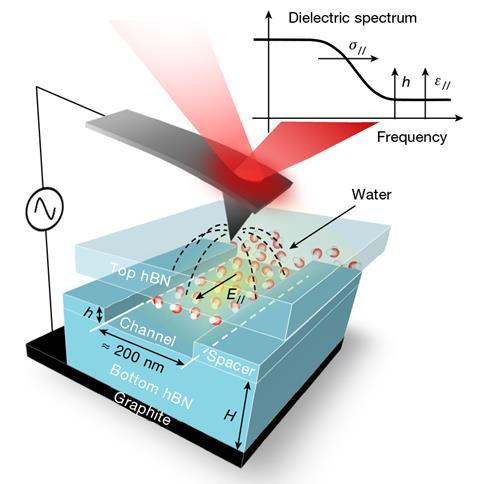
In the new work, the researchers modified the setup slightly, scanning the tip across the surface at a wide range of frequencies to measure the conductivity and dielectric constant along the channel. They found that the two increased in tandem and reached startlingly high values. The dielectric constant increased tenfold at around 2nm width, and the conductivity peaked at over five orders of magnitude higher than in bulk water, before decreasing again slightly as the channel narrows further.
These findings suggest the hydrogen bonding becomes quasi-2D, with the water forming a layered structure inside the nanochannel. The details are not entirely clear, but the conductivity peaks at the channel diameter at which models predict that the two interfacial water layers just touch ie at the onset of strong confinement. The peak conductivity value is comparable with that of commercial proton exchange membranes, raising the intriguing possibility that nanoconfined water may have similarities to superionic ice. The researchers are now investigating further to ascertain whether water behaves similarly in nanochannels made from other materials.
‘This is a great development,’ says biophysicist Sonia Contera at the University of Oxford, who says the findings underline how poorly interfacial water is understood. She says the 2018 work was the subject of severe pushback from other researchers in the microscopy community who refused to believe the results. ‘There’s a ton of work here,’ she says. ‘With this paper, [Fumagalli and her colleagues] win.’ Beyond this, she says, the work ‘opens possibilities that biological systems are able to create proton highways… You start to control very interesting physics not just for biological systems but also for future nanotechnological devices, non-conventional computing and so on’.
References
R Wang et al, Nature, 2025, 646, 606 (DOI: 10.1038/s41586-025-09558-y)















No comments yet