Sugar chemists have developed a highly efficient synthetic pathway to produce a variety of oligosaccharides from scratch. While there are numerous options in a glycochemists’ toolbox to produce monosaccharides, automated synthesis of oligosaccharides is still very limited. The new approach not only complements conventional methods but allows the synthesis of unnatural oligosaccharides, which could be important in biological and medicinal chemistry studies.
The essential role of complex carbohydrates in biological systems is becoming increasingly apparent, such as in protein-protein interactions and cell signalling. Multi-step or semi-synthesis can access natural sugars; however, both the D- and L-sugars are required to differentiate between the contribution to the physical properties (such as having a sugar-protein scaffolding role) and site of action or molecular recognition to the observed biological effect.
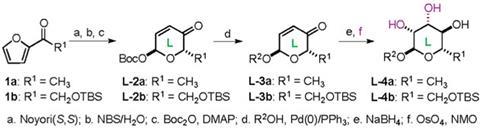
The addition of extra sugar building blocks is controlled by the double bond of the pyranone, which works with a palladium catalyst to direct the glycosylation. The enone functionality of the pyranone serves as an atom-less protecting group for three of the potential hydroxyl groups, and therefore reduces the number of protection–deprotection steps. ‘These protection–deprotection steps are often more laborious than the carbohydrate synthesis,’ says O’Doherty. ‘Our approach is quite orthogonal to these [conventional] approaches and has the significant environmental advantages of using non-alcohol functional groups (alkenes and ketones) as atom-less protecting groups.’
The team were able to make a highly branched, unnatural (all-L) hepta-oligo-mannoside in just 12 steps from an achiral acylfuran starting material. ‘What is unique about our approach is how it allows us to also prepare the unnatural analogues,’ says O’Doherty. ‘We obviously prefer our approach because it minimises the use of wasteful protection–deprotection steps and shows the power of asymmetric catalysis.’
Chemical glycobiology expert Rob Field from the John Innes Centre, UK, says: ‘We spend a huge amount of time playing around with protecting groups. One of the nice features of the work from O’Doherty is that, basically, that is avoided by not starting from a sugar building block and only putting in the functionality after the monomeric building block is stitched together. It’s a radically different approach and I think it offers a lot of potential.’
‘Our next big step is getting the glycobiology community to start thinking that this approach is a reasonable tool in their synthetic tool box,’ says O’Doherty. ‘We believe this de novo approach will allow glycobiologists to posit medicinal chemistry-like questions in their systems of interest.’
References
- R S Babu et al, J. Am. Chem. Soc., 2012, 134, 11952 (DOI: 10.1021/ja305321e)






No comments yet