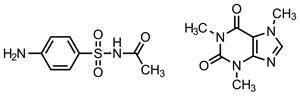
Sulfacetamide (SACT) is often lost on blinking and in tears when applied as a treatment for conjunctivitis and other ocular ailments. This leads to the inconvenience and complications of applying larger and more frequent doses of SACT.
Various schemes have been investigated, including trapping SACT in bioadhesive microspheres, to slow drug release and prevent its washout. But these either limited the drug’s bioavailability or weren’t suitable to market.
To solve the problem, Ashwini Nangia and colleagues at the University of Hyderabad in India, looked to cocrystals. Crystallising an existing drug with another safe substance can change the physicochemical properties of a medicine without having to change the drug molecule itself.
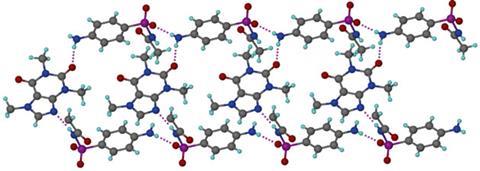
The team reasoned that replacing weaker hydrogen bonds in the crystals with stronger ones could lower the crystal’s solubility and dissolution rate. A selection of molecules were therefore cocrystallised with SACT. Caffeine proved to be one of the most successful, making the drug less soluble and the crystals denser – suggesting the molecules packed tighter together – compared to SACT alone. After studying the hydrogen bonding in SACT and in the cocrystal, stronger N–H---O bonds in the latter (compared to C–H---O) were found to cause the denser crystal packing and lower solubility.
Srinivasulu Aitipamula, a pharmaceutical cocrystal expert at the Institute of Chemical and Engineering Sciences, Singapore, explains that the the identification of intermolecular interactions which can be altered by cocrystal formation is what makes this research stand out. ‘Whereas such design strategies are very well established, the fact that the same strategies have been applied to a less explored area of reducing the solubility/dissolution rate of the drug for improved therapeutic action makes this study noteworthy.’
References
This paper is free to access until 12 May 2014. Download it here:






No comments yet