Encouraging prospects for eco-friendly pyrotechnics and propellants based on nitrogen
Lewis Brindley/Turin, Italy
Eco-friendly explosives based on nitrogen compounds could soon compete with conventional detonators and propellants used in pyrotechnics, mining, and military applications, according to scientists speaking at the 2nd EuCheMS Chemistry Congress in Turin, Italy.
In work presented from Thomas Klap?tke’s group at the University of Munich, Carles Mir? Sabat? explained that the team have developed simple ways to make nitrogen-based explosive compounds such as 5-nitrotetrazole salts, [1] and are using some of them to make lead-free detonators, reducing the environmental impact of military explosives.[2]
The latest research follows ongoing efforts to make compounds that are safer to handle, explode more cleanly, and produce fewer toxic byproducts and smoke particles than today’s popular explosives (such as TNT) or rocket propellants (such as perchlorate, a potential human health hazard).
Klap?tke’s explosives are based on tetrazoles, 5-membered rings containing four nitrogen atoms and one carbon atom. After making structural tweaks, such as adding nitro groups, the researchers say the compounds can be made to explode with comparable power to explosives like TNT and RDX.
But tetrazoles are far less sensitive to shock or impact, allowing them to be handled more easily. And their explosions are much cleaner, producing mostly nitrogen gas instead of the smoky byproducts of incomplete combustion of carbon-based compounds. The compounds are also non-toxic, so remains will not contaminate nearby land or water supplies.
To compete with well-established explosives, however, tetrazole compounds have to be finely-tuned, and cheap. ’We are trying to find compounds with high performance, low sensitivity and good stability under a wide range of conditions,’ Sabat? told Chemistry World. ’These properties are often mutually exclusive, so finding one that works across all areas is a bit like hunting for drug candidates in the pharmaceutical industry.’
The tetrazole structure is relatively easy to modify: adding extra alkyl groups, for example, grants more stability to an explosive. So Klap?tke’s group have developed a number of tailored compounds for specific applications, from detonators to rocket fuel. Most recently, they have demonstrated simple ways to make several tetrazole explosives from the relatively cheap starting material of 5-amino tetrazole.[1] All these processes are performed at low temperature and have the potential to be scaled up in future, Sabat? said, although careful handling is required.
Ready, aim, fire
Klap?tke’s team have recently been investigating the use of tetrazolium salts as a replacement for lead azide, an explosive used in detonators to prime pistol ammunition and to help set off tank shells. Switching to nitrogen-based detonators would reduce soldiers’ exposure to toxic lead emissions and keep gun barrels clean, reducing the chance of weapon systems jamming. The cleaner explosives would also cut down on smoke emissions that might betray a soldier’s position.[2]
Nitrogen explosives are being considered in fireworks, too. The distinctive red colour of strontium perchlorate makes it a pyrotechnic favourite, but the perchlorate decomposes into hydrochloric acid and soot, producing dusty clouds that can be harmful to health.[3] Switching over to using tetrazolium salts would give much cleaner and brighter explosions, Sabat? says.
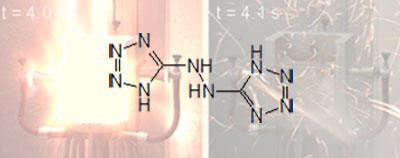
Beyond explosions, the team are also investigating the use of tetrazoles as propellants. Earlier this year the team linked two tetrazole rings together via a hydrazine group, resulting in a bistetrazole currently under investigation for use as rocket fuel.[4] A chemical explosive like this could be cheaper and safer than the currently-used mix of liquid hydrogen and oxygen, and it avoids the environmental concerns of other chemical propellants such as ammonium perchlorate.
’This is an important area of research,’ said Gerhard Lammel from the Max Planck Institute for Meteorology in Germany, who chaired the session on air pollution in Turin. ’However, we don’t really know just how serious the environmental impacts of explosives are at the moment,’ he said, indicating that the industry could be slow to switch to a greener alternative.
Enjoy this story? Spread the word using the ’tools’ menu on the left.
References
et al, New Journal of Chemistry,et al, Dalton Trans.et al, J. Environ. Monit.,10, 979.
4 C Miró Sabaté et al, Chem. Mater. 2008, 20, 3629






No comments yet