Computer models that show how organic molecules could assemble into molecular quasicrystals may open the door to new materials with exotic properties.1
Imitating the famous mathematical patterns known as Penrose tilings, Dimitri Laikov of Moscow State University in Russia, designed two complementary molecular ‘tiles’ and modelled their supramolecular interactions. The resulting assembly shows the aperiodicity and five-fold rotational symmetry characteristic of quasicrystals – the first time that a complex with this property has been predicted.
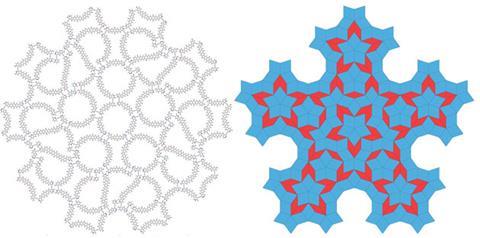
Quasicrystals were discovered in the 1980s but it took many years for them to be accepted as they break many of the conventional rules of crystallography. Unlike regular crystals which have translational symmetry and are limited to 2-, 3-, 4- and 6-fold rotational axes, quasicrystals can have 5-fold and 10-fold rotational symmetry and no translational symmetry at all.
Hundreds of materials have now been found with quasicrystalline phases. However, unlike conventional crystals which can form from nearly any material from salt to proteins, almost every known quasicrystal is a three-component metallic alloy such as the mineral icosohedrite, Al63Cu24Fe13. This raises the possibility that a vast number of quasicrystals formed from much more complicated compounds remain undiscovered.
Zhongfu Zhou and Kenneth Harris of Cardiff University, UK, were the first to report the design of a molecular quasicrystal, in which the organic molecules represented the nodes of the Penrose tiling.2 Laikov took an alternative approach by designing his components to mimic the thick and thin ‘rhombs’ that are present in the Penrose tiling, using tactically-placed hydrogen-bonding groups to ensure that they bond to each other in the orientations required produce a quasicrystal. ‘Many molecules I tried failed to build the beautiful tilings I wanted,’ says Laikov, ‘but through hard work I found molecular pairs that passed all the tests.’
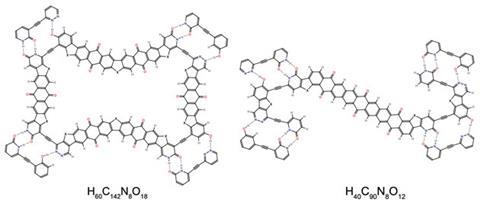
Speculating on the applications of his research, Laikov says the molecular quasicrystals may have unique features as their hierarchical structure could lead to unusual optical, mechanical, electromechanical and thermal properties. ‘Even if no practical applications are found, their aesthetic pleasure may inspire further daring designs of new molecular materials,’ he adds.
‘Reading Laikov’s work, I was reminded of the enormous progress of computational chemistry,’ says computation chemist Georg Schreckenbach of the University of Manitoba in Canada. ‘Predicting new molecules, and especially those that are beautiful or show novel bonding patterns, has always been a holy grail for computational chemistry.’
Schreckenbach goes on to say that the research reaffirms how aesthetics, beauty and mathematical elegance often serve as the driving force behind scientific discovery: ‘One can sense the pure joy of discovery that was clearly the motivation for this study.’
Laikov is now planning to synthesise the molecules he has modelled to test the formation of the complexes in the laboratory.
References
- D Laikov, RSC Adv., 2014, 4, 17925 (DOI: 10.1039/c4ra01354a) (This paper is free to access until 3 June 2014.)
- Z Zhou and K D M Harris, ChemPhysChem, 2006, 7, 1649 (DOI: 10.1002/cphc.200600278)






No comments yet