Counterfeit medicines can kill - so shouldn't we lock up the people producing them? Bea Perks finds out it's not quite that simple
Counterfeit medicines can kill - so shouldn’t we lock up the people producing them? Bea Perks finds out it’s not quite that simple
Medicines would seem to lend themselves to conmen for two reasons. First, you can’t see what you’re buying or, at least, you can’t see what is inside the product you are buying. Drug industry laboratories aren’t filled with great lumps of analytical equipment for aesthetic reasons. They are there to work out what is going on at the molecular level. Without them you are shopping blind, like buying a car without lifting the bonnet to check there’s an engine inside. This means sales are made primarily on trust - music to the ears of any con artist.

Second, there’s a lot of money involved. According to an October 2010 report from market intelligence company IMS Health, the global pharma market will top $880 billion (?563 billion) in 2011. Siphon off even a tiny fraction of that and you’re still talking about huge financial motivations.
As a result, and despite the best efforts of Pfizer and others, drug counterfeiting has become an enormous and rapidly-growing problem. The Center for Medicines in the Public Interest, a US-based non-profit medical issues research group, predicts that counterfeit drug sales will have hit $75 billion globally in 2010, an increase of more than 90 per cent from 2005.
A conman’s market

Data is difficult to get hold of, with current figures buried in a wide variety of places, including: reports from non-governmental organisations, pharmaceutical companies and national drug regulatory and enforcement authorities; ad hoc studies conducted on specific geographical areas; and occasional surveys. But it is clear that developing countries - where the need for drugs is often highest and the ability to pay lowest - are the worst affected. The World Health Organization (WHO) estimates that sales of counterfeit drugs range from between around 1 per cent of total sales in developed countries to over 10 per cent in developing countries. Counterfeiting is unsurprisingly greatest in regions where regulatory and legal oversight is weakest. Many countries in Africa and parts of Asia and Latin America have areas where more than 30 per cent of the medicines on sale are counterfeit, while other developing markets have less than 10 per cent.
Most industrialised countries with effective regulatory systems and market control, including the US, most of the EU, Australia, Canada, Japan and New Zealand, have a lower proportion of pharmaceutical fakes - less than one per cent of market value. Nevertheless, Pfizer - makers of the heavily counterfeited drug, Viagra - unearthed a €10.5 billion (?9 billion) black market across 14 European countries (including the UK, Germany, France and the Netherlands) during a research project - Cracking Counterfeit Europe - carried out at the end of 2009. The researchers found that, of 14,000 people surveyed across those countries, one in five would admit to buying prescription only medicines from illicit sources. The reasons they gave for taking this route? Cost and ease of access.
A tangled web

The WHO first addressed the issue of counterfeit medicines at the international level in 1985 at the Conference of Experts on the Rational Use of Drugs in Nairobi, Kenya. The meeting recommended that the WHO, together with other international and non-governmental organisations, consider setting up a clearing house to collect data and to inform governments about the nature and extent of counterfeiting. Since then, a dramatic increase in international trade of pharmaceuticals and sales via the internet has significantly boosted the counterfeit market. (How many emails promoting cut-price V!*gra have appeared in your junk email folder?). Prompted by this, the WHO launched the International Medical Products Anti-Counterfeiting Taskforce (Impact) in 2006. Medicines purchased over the internet from sites that conceal their physical address are, according to the WHO, counterfeit in over 50 per cent of cases.
But the challenge of combating drug counterfeiting is hideously complex. The lifecycle of a drug - from a collection of raw materials to the finished product in a glossy packet - provides ample opportunity for fakers, smugglers and various assorted ne’er-do-wells to step in. Even defining a ’counterfeit medicine’ is tricky.
In 2007, the WHO Impact programme issued a set of ’principles and elements for national legislation against counterfeit medical products’, in which the term was defined (see What makes a fake? p58). In November 2009, the director-general of the WHO sent out a circular letter inviting member states to provide information on how the term is used in national legislation and to provide comments on the definition drawn up by Impact. As a result, considerable variety among national definitions of ’counterfeit medicines’ has been unearthed, something echoed in an ongoing WHO survey of legislation.1
The list of inconsistencies is a lengthy one. While some countries have incomplete definitions, others have no definition at all. Some countries consider the counterfeiting of medical products a serious crime. Others consider it a minor crime - a lesser crime than counterfeiting T-shirts even - and others don’t consider it a crime at all. In some cases, sanctions are not applicable unless it is proven that counterfeits have resulted in injuries or deaths, and in many cases the responsibilities of those involved in the distribution system are not clearly defined. Some countries don’t even use the term counterfeit, opting instead for falsified, illicit, illegal, unregistered, unauthorised, spurious or adulterated. The lack of standardisation makes international co-operation difficult and plays into the hands of the criminal masterminds behind genuinely dangerous, life-threatening counterfeit medical products see table.
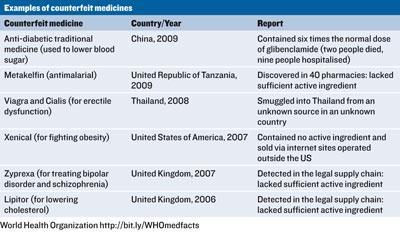
The issue is further complicated by recreational use of prescription drugs, such as Viagra, which adds unique legal and medical issues. For a start, recreational users tend to be less concerned by possible risks as they are already knowingly taking risks by using the products. Furthermore, they attract less sympathy from politicians and lawmakers. Probably because of this, they are more likely to be at risk from seriously sub-standard and potentially life-threatening counterfeits.
International action
Despite the complexities, the international community seems newly motivated to combat the problem. In November 2010, members of the European parliament (MEPs) passed a resolution welcoming the newly completed but not yet signed anti-counterfeiting trade agreement (Acta). MEPs hailed the agreement ’a step in the right direction’, but called on the Commission ’to confirm that Acta’s implementation will have no impact on fundamental rights and data protection, on the ongoing EU efforts to harmonise intellectual property rights enforcement measures, or on e-commerce.’ In response to an earlier draft of Acta, issued in March 2010, a majority of MEPs had agreed that Acta should not harm global access to legal, affordable and safe medicines.
In a briefing paper published in November 2010 by the British foreign affairs and security think-tank Chatham House, independent consultant Charles Clift cites the example of recent EU detentions of generic medicines. There were at least 19 such detentions between 2008 and 2009, mostly in the Netherlands and mostly involving drugs that had been manufactured in India and were en route to non-EU countries, including Brazil.
The detentions took place under an EU regulation introduced in 2003 that permitted action against goods infringing intellectual property (IP) rights, including goods in transit.2 In most cases, says Clift, the detentions appeared to be on the grounds of suspected patent infringements in the Netherlands, even though the products were not patent protected in either India, where the drugs were made, or their destination countries.
In May 2010, India and Brazil each submitted to the World Trade Organization (WTO) a ’request for consultations’ with the EU and the Netherlands. Requests of this kind are the first step in the WTO dispute settlement process.
According to Clift, the requests elaborate the various ways in which EU and Dutch laws and regulations may be contrary to the WTO’s general agreement on tariffs and trade (Gatt) and trade-related aspects of intellectual property rights (Trips). The EU previously argued that the regulations under which the detentions were made were compatible with Trips, and that confiscating genuinely counterfeit medicines in transit was beneficial to countries outside the EU, which would probably have less capacity for identifying them.
If an agreement can’t be reached, it could take a dispute settlement panel up to year to issue a report. Meanwhile, the EU has launched a consultation on its relevant regulation with a view to making possible amendments, one aspect of which relates to the treatment of goods in transit.2
In June 2010, the US government’s intellectual property enforcement coordinator released a joint strategic plan on IP enforcement. The plan established a Counterfeit Pharmaceutical Interagency Committee to focus on ’robust enforcement’ of intellectual property rights worldwide with the goal of ’enhancing US advocacy on IP issues in multiple international forums.’
Pharmaceutical Research and Manufacturers of America (PHRMA), which represents leading pharmaceutical research and biotechnology companies in the US, welcomes the plan. ’While IP infringement threatens the ideas and innovations that are the lifeblood of pharmaceutical companies and in turn our industry’s important stake in the US economy, it also presents a very real public health and safety danger to patients worldwide,’ says PHRMA president, Billy Tauzin. The organisation is reviewing the plan and is in ongoing dialogue with government stakeholders and others on these and alternative mechanisms that could help reduce and eliminate the presence of counterfeit drugs worldwide.
What makes a fake?
According to the WHO definition,1 a medical product is counterfeit when there is a false representation in relation to its identity,2 history or source.3 This applies to the product, its container or other packaging or labelling information. Counterfeiting can apply to both branded and generic products. Counterfeits may include products with correct ingredients or components,4 with wrong ingredients or components, without active ingredients, with incorrect amounts of active ingredients, or with fake packaging.
References
1 Taken from the International Medical Products Anti-Counterfeiting Taskforce (Impact)’s ’Principles and elements fornational legislation against counterfeit medical products’
2 For example, any misleading statement with respect to name, composition, strength, or other elements
3 For example, any misleading statement with respect to manufacturer, country of manufacturing, country of origin, marketing authorisation holder
4 This refers to ingredients or any other component of a medical product
The industry response
The counterfeit medicine market is worth billions worldwide, money that is being stripped out of the pharmaceutical industry either through erosion of sales or loss of reputation.
On one front, companies try to work out what’s real and what isn’t. Pfizer has been a pioneer in this area, opening a dedicated forensic lab in 1998 at its site in Sandwich, UK. The lab, led by development chemist Wendy Greenall, starts by analysing the drug formulation with infrared spectroscopic techniques. ’Often you can tell straight away that it’s not the same formulation because it has a different fingerprint,’ says Greenall. Chromatographic techniques and powder x-ray diffraction are also routinely used. Even if a drug is clearly counterfeited, its exact formulation might be needed to assist in prosecutions. ’Then we all enjoy using more unique techniques,’ she says, such as direct analysis in real time (DART) mass spectrometry. Greenall’s team makes regular appearances in courts in Africa and the Middle East, although in the face of the data many accused parties admit guilt.

On another front, companies look to educate consumers. They have responded to the threat of counterfeiting with a series of massive campaigns. The problem, note Peggy Chaudhry and Stephen Stumpf at the Villanova School of Business in Philadelphia, US, is a lack of focus. ’Companies have tried everything from threatening prosecution to linking phony products with organised crime’, they write in the Massachusetts Institute of Technology journal, MIT Sloan Management Review . ’But marketers often don’t pay attention to what actually drives people in particular markets to buy counterfeits and what messages will actually work to curb demand of fake goods.’
Chaudhry told Chemistry World: ’We performed a web survey in Brazil, Russia, India, China and the US about consumer complicity to obtain fake pharmaceuticals to better understand why a person engages in this illicit behaviour.’ Their results suggest that consumers from different countries are motivated by different factors. According to the findings, Russian consumers for example are not particularly motivated by whether a counterfeit drug shares the quality and performance of the legitimate product. It is more important that the product is cheaper and easier to get hold of. In Brazil, India and the US, consumers responded that consumption of fake drugs was unethical, but the question of ethics does not appear to worry consumers in Russia and China. The disparity underlines the need for targeted responses from the pharmaceutical sector. There is no point building a worldwide campaign around a factor that only bothers consumers in one or two countries.
Chaudhry welcomed Pfizer’s recent ’regurgitated rat campaign’ in the UK, which warned consumers about the risks of obtaining drugs via the internet. An unwise consumer is shown taking a counterfeit drug that causes him to - you’ve guessed it - regurgitate a rat. The film is designed to alert consumers to the possible inclusion of rat poison in some counterfeit drugs.
According to Pfizer, the advert, released in 2009, led to an increased awareness of the risks of counterfeit medicine in the UK. The company has used different campaigns - at least one other incorporating a rat - in other countries.
Steve Allen, Pfizer’s head of global security, joined after a period fighting counterfeits at global software company Microsoft. Before that he worked with the UK National Crime Squad (now the Serious Organised Crime Agency). The firm works closely with the UK government’s Medicines and Healthcare Products Regulatory Agency (MHRA) and Allen says the company is focused on forging relationships with national agencies worldwide. When a counterfeit is spotted, the relevant agency is alerted. In return, national agencies get in touch with Pfizer when they spot suspicious products.
Clearly, the best results will come through cooperation. Last year, the Tanzania Food and Drug Authority (TFDA) found what they suspected was a sub-standard batch of the malaria drug metakelfin. Allen says that Pfizer sent its top forensic chemist, who found that the wrong test was being used. ’Pfizer overturned the TFDA decision,’ he recalls. The drug went back on sale shortly afterwards, with the addition of improved, tamper-free packaging.
But such successes belie the scale of the problem, which is unlikely to ever be resolved fully. Most counterfeit drugs are made in India and China, says Allen, and sadly many of the markets are in Africa, where the medical needs are greatest and the ability to outmanoeuvre the counterfeiters is lowest. Allen is candid about the chances of those looking to dodge the phoney chemistry. ’They don’t have a cat in hell’s chance,’ he says.
References
1 WHO Preliminary draft survey on national legislation on "counterfeit medicines"
2 Council Regulation (EC) No 1383/2003 of 22 July 2003 concerning customs action against goods suspected of infringing certain intellectual property rights and the measures to be taken against goods found to have infringed such rights






No comments yet