Antarctica may seem pristine and almost devoid of life, but there’s plenty of chemistry going on. Victoria Atkinson explains what it can tell us about the climate and pollution across the globe
-
Antarctica as a climate archive: Ice and sediment cores from Antarctica provide a detailed record of Earth’s atmospheric and environmental history. Scientists analyse trapped air bubbles and organic compounds to reconstruct past climates and trace global events like wildfires, glaciation, and human activity.
-
Oxidation and atmospheric chemistry: Atmospheric chemist Chiara Giorio studies oxidation products from plant terpenes in ice cores to infer historical levels of atmospheric oxidants like hydroxyl radicals and ozone. These insights help understand how Earth’s natural ‘cleansing’ capacity has changed over time.
-
Sea ice and climate feedback: Marine sediment studies using biomarkers from algae reveal how seasonal sea ice has changed over the last 40,000 years. Findings suggest that sea ice retreat may have triggered deep ocean changes and carbon dioxide release, contributing to past global warming.
-
Pollution accumulation and future risks: Despite its remoteness, Antarctica is accumulating persistent organic pollutants through atmospheric transport. Researcher Cristóbal Galbán-Malagón warns that warming could turn Antarctica from a pollution sink into a source, releasing stored toxins back into the environment.
This summary was generated by AI and checked by a human editor
Antarctica is often described as the last untouched place on Earth. First sighted in 1820, the ‘International Continent’ is now a protected area for collaborative scientific study, acting as a bellwether for the health of the planet. Early explorers began establishing research bases over 100 years ago and today there are more than 70 permanent stations scattered across the continent.
Ice core analysis is the best known of these research activities – the steady and undisturbed accumulation of snow over the continent’s 34 million-year history trapped bubbles of air between ice layers, creating a continuous record of how the planet’s atmosphere has changed through the millennia. Polar researchers drill deep into the ice, harvesting cores of around 10cm in diameter and often totalling several kilometres long. They then meticulously analyse individual slices of each core, using a combination of theoretical models, isotopic signatures, and seasonal changes to date each sample. ‘In those bubbles we can see what was in the atmosphere thousands of years ago, for example greenhouse gases like carbon dioxide and methane,’ says Chiara Giorio, an atmospheric chemist at the University of Cambridge, UK. ‘But the ice around the bubbles is also old and contains a lot of information: inorganic compounds like sea spray, sulfates from volcanic emissions, and also organic compounds.’
Organic indicators
In the barren icy landscape of Antarctica, it’s easy to dismiss the significance of organic chemistry. Paleoclimatologists generally agree that falling temperatures at the end of the Eocene period around 34 million years ago triggered the formation of the first ice sheets, largely excluding plant and animal life and suppressing the natural cycles of carbon-based compounds.
Organic molecules are ubiquitous – and reactive
But despite the remoteness of the continent, exacerbated by its isolating circumpolar current, even trace compounds released elsewhere on the globe make their way to the Antarctic one way or another. ‘Organic molecules are ubiquitous, not just in the atmosphere, but also in environmental matrices like water, soil and living organisms,’ explains Giorio.
Consequently, there is a huge research drive to understand the changing levels and shifting cycles of these organic compounds and how they have impacted, and continue to impact, the Antarctic environment and wider planet. Through careful choice of organic proxy, it’s possible to identify the species present at a particular time, pinpoint global environmental events like wildfires or glaciation, and more recently, to track human activity. ‘We can also look at processes. Organic compounds tend to be reactive which can be very interesting for understanding what kind of processes in an environment are modifying the chemistry of those compounds,’ adds Giorio.
Atmospheric cleaners
Giorio’s work focuses specifically on the process of oxidation. The Earth’s atmosphere is naturally oxidising and photochemical cycles within the stratosphere and troposphere protect the surface from UV radiation and regulate the levels of trace gases such as methane and nitrous oxides.
‘The oxidising capacity is determined by two main compounds in the atmosphere: the hydroxyl radical and ozone. The hydroxyl radical removes methane very efficiently and reacts with a lot of other organics, so we can see it as a sort of cleanser of the atmosphere,’ she explains. ‘But on the other side, there is the formation of aerosols from the oxidation of gaseous compounds, so it can also contribute to pollution in other areas by producing more condensed organic compounds.’
The levels of these two oxidising agents therefore dictate the lifetime of almost every other component within the atmosphere, which in turn influences the distribution and transport of pollutants like volatile organic compounds (VOCs), per- and polyfluoroalkyl substances (PFAS), and particulate matter around the planet. Consequently, as human activity continues to alter the composition of the atmosphere, understanding how these cleansing cycles are changing will enable us to mitigate against the evolving impacts of anthropogenic chemicals.
Both ozone and hydroxyl radicals are too reactive to persist within the atmosphere and it’s not possible to directly measure either via ice core analysis. However, their influence on more long-lived molecules can be used to infer the likely oxidising activity at different points in the planet’s history. But choosing a suitable proxy for such a generalised and interconnected process is immensely complex. ‘If the proxy is affected by other environmental factors, that breaks the correlation between the proxy and what you’re trying to observe,’ says Giorio. ‘You either need a chemical marker that only responds to a single environmental parameter or you need to start thinking of a more complex way of getting that information.’
Giorio has opted for the latter strategy and works with a panel of oxidation products derived from the plant terpene α-pinene. This volatile and fragrant compound emitted by conifers as a defensive mechanism against insect pests and is subsequently oxidised by ozone and hydroxyl radicals to form a series of secondary aerosols including cis-pinonic acid, pinic acid, and nopinone . From the vast array of compounds in this reaction scheme , Giorio carefully selected a panel of 10, each formed by a different sequence of oxidative steps and sufficiently stable to survive atmospheric transport to the Antarctic.
The current modelling studies don’t agree
The team then developed specialist mass spectrometry techniques to detect the minute quantities of the chosen proxies trapped in the water from melted ice core samples. By comparing the ratios of different oxidation products against the original plant terpene and evaluating the relative rates of the different steps in this complex interconnected web, they can then calculate backwards to determine the oxidising capacity at the time of each core sample.
Giorio is now building up a bank of empirical data using these different oxidative proxies and hopes to ultimately compare the final results against existing theoretical models for the Earth’s oxidising capacity. ‘These current modelling studies don’t agree which is why this is so interesting,’ she says. ‘There are some that are based on photooxidation – how the hydroxyl radical is produced and removed from the atmosphere – that suggest the oxidising capacity hasn’t changed much. But there are other models based on the lifetime of methane that suggest there was a 20% difference, so it’s very much an open question.’
Tracking the ice
Organic proxies are not just restricted to the ice. The marine sediments surrounding Antarctica contain a rich repository of biomolecules which can help trace ecological activity back through the millennia. Similar to ice core extraction, sediment cores are obtained by boring into the seabed with a metal pipe to obtain thin lengths of compressed sediment, laid down over thousands or even millions of years. Smaller sections are then processed separately to extract the particular components of interest, whether that’s fossilised shells, mineral samples or organic matter.
During a two-year stint at the Australian National University, Henrik Sadatzki, now based at the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research in Germany, specifically focused on a pair of branched isoprenoids, IPSO25 and HBI III , produced by different sea algae to reconstruct the extent of seasonal sea ice over the last 40,000 years. ‘There’s a diene (IPSO25) which has been found to be produced by sea ice algae around Antarctica and we combined this with another biomarker, a triene (HBI III), that’s mainly produced by open-water diatoms,’ he explains. As photosynthesising organisms, both plankton species principally produce these biomarkers during spring and summer, enabling Sadatzki’s team to reconstruct the changes in seasonal sea ice coverage from the relative abundance of each compound in the sediment core samples.
The broad results of this study were exactly as predicted: higher concentrations of the diene indicated that sea ice dominated even in the summer season until around 20,000 years ago (the last glacial high), after which time the open-water triene biomarker began to increase as the world entered its current interglacial period.
However, the timeline of this transition as evidenced by the two isoprenoids supports an interesting emerging hypothesis explaining what may have driven this latter warming. ‘One finding that we had suggested that the sea ice during spring and summer had already started to decline before other major changes happened on a global scale, like the atmospheric carbon dioxide increase and the main global warming during the deglaciation,’ says Sadatzki.
The chronology of these events has extremely important implications for the mechanism of warming, he continues. ‘Other studies show that the chemical composition of deep abyssal waters in the Southern Ocean showed a major transition across the deglaciation, also starting when we observe the sea ice retreat. That indicates changes in the deep stratification of the Southern Ocean, which is an important factor for storing carbon dioxide.’
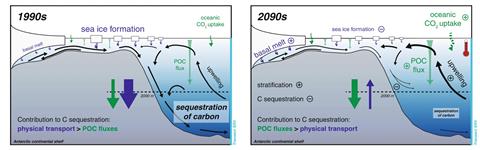
During the last glacial maximum, ice core records indicate that atmospheric carbon dioxide was around 90ppm lower than pre-industrial levels, and models suggest that large parts of this missing carbon dioxide were stored in the deep Southern Ocean, with stratified currents and the sea ice ‘lid’ preventing the exchange of stored gas with the atmosphere. But paleoclimatologists hypothesise that the seasonal cycle of the ice itself may also have contributed to this gas-sequestering effect.
The formation of sea ice releases brine, creating very dense water near the coast. This sinks down to the sea floor, taking the dissolved carbon dioxide with it. Meanwhile, the density contrast between it and the water above results in stratification, which then traps the stored carbon dioxide at the bottom. But as seasonal sea ice retreats, the smaller release of brine forms less dense water, weakening the stratified storage effect. Simultaneously, the open sea surface allows the resulting upwelling waters to release stored carbon dioxide into the atmosphere.
The complex interplay of these changes is difficult to disentangle but for Sadatzki, the first step in preparing for the future has to be understanding the past. ‘If you want to argue anything about how man-made climate change is affecting the global climate, we need to understand the natural processes,’ he says.
A pollution sink
While Giorio and Sadatzki are using chemicals of the past to understand the present and map out the future, Cristóbal Galbán-Malagón is exploring completely new territory. His research at Universidad Mayor in Santiago, Chile, focuses on Antarctic pollution: how chemical contaminants are transported and stored in the Antarctic and the impact of these compounds on the ecosystem once there.
Persistent organic pollutants, industrially produced toxic compounds with long lifetimes and slow degradation rates, are one such group of chemicals. Many, including the insecticide DDT, the fungicide HCB, and the electronics additive PCB are now banned and, since the Stockholm convention in 2001, the use of similar hazardous additives in Europe has been steadily falling. Yet despite a sustained decline in production, continuous long-range transport of these semi-volatile compounds means levels are still rising in the formerly pristine Antarctic.
Part of this is due to an effect called global distillation, explains Galbán-Malagón. Small quantities of more volatile compounds like PCB-28 spontaneously evaporate and are transported through the atmosphere via air currents. However, on reaching the cold air mass around the Antarctic continent, these compounds rapidly condense and precipitate with snowfall or as particulate matter. ‘Another proven hypothesis is the grasshopper effect which states that semi-volatile compounds do successive steps of hopping like a grasshopper. So they volatilise, travel in the atmosphere, deposit, then re-volatilise, travel again, and deposit,’ he explains. ‘Finally they reach the Antarctic area and, with these cold temperatures, they condense – but cannot re-volatilise so it becomes their final sink.’
A proportion of these pollutants is immediately trapped in the snow, with the remainder entering the carbon cycle via lipophilic interactions with organic matter such as soil or dead organisms. ‘Some of these compounds reach the sediments in the deeper waters because they are attached to these organic particles. It’s called marine snow where this complexation of recalcitrant organic matter passes down through the water column,’ says Galbán-Malagón. However, an alarming quantity also make their way into the food chain, slowly poisoning the ecosystem and accumulating in larger organisms like seals and whales. And worryingly, Galbán-Malagón believes this problem is only likely to get worse.
‘Antarctica will probably go from a sink to a source. Everything is a chemical equilibrium with environmental matrices and with increasing environmental temperatures and the ice melting, these compounds may be released and the equilibrium will be displaced,’ he says. ‘The next question is, will these compounds remain in Antarctica or begin to migrate back into the world?’
Scientists are already beginning to see a shift in certain areas of the continent with the net movement of persistent organic pollutants moving away from storage in snow and sediments and closer to an equilibrium position. Antarctica’s isolating air currents make global recontamination unlikely in the next few hundred years, but ‘this is a red flag about what is going to happen’, warns Galbán-Malagón. ‘The concentrations of these pollutants will increase but we cannot predict the amount that will be released.’
Significant environmental changes as a result of global warming are now inevitable but Antarctic research will be a key tool preparing us to meet these challenges and mitigate against the worst impacts. ‘We learn a lot more than we think from Antarctic areas. We know about chemical releasing, we know about invasive species, we know about climate change and impacts on ice melting,’ says Galbán-Malagón. ‘So to protect this area and keep researching, I think it’s mandatory for human development.’
Victoria Atkinson is a science writer based in Saltaire, UK





















No comments yet