As we look forward to the 2008 Nobel prizes, Mike Sutton recalls the work of two scientists who redefined chemistry's disciplinary boundaries
As we look forward to the 2008 Nobel prizes, Mike Sutton recalls the work of two scientists who redefined chemistry’s disciplinary boundaries
As our stock of scientific information grows, the degree of specialisation within science tends to increase. Students heading for the frontiers of knowledge are encouraged to learn more and more about less and less. But in every generation, adventurous individuals have resisted this trend, sometimes with world-shaking results.
This month we celebrate two chemistry Nobel Prize-winners - Ernest Rutherford (1908) and Frederick Sanger (1958) - who transcended traditional disciplinary boundaries. Both of them grew up in small rural communities and refined their research skills in Cambridge laboratories. (And both became intimately acquainted with potatoes early in their careers.)
Rutherford connected physics with chemistry at a fundamental level. Most 19th century chemists had carefully avoided speculating about what atoms actually were, concentrating instead on the patterns in which they combined. Rutherford’s work opened the way to an explanation of chemical combination which was grounded in the physical structure of atoms themselves.
Sanger developed innovative chemical techniques that revolutionised biology. When he started work, the molecular structures of proteins and nucleic acids - and their functions inside living organisms - were still poorly understood. As an individual researcher, and as a team leader, Sanger played a crucial part in cracking key molecular codes which set the parameters for life on earth.
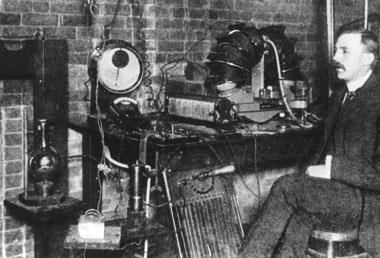
Ernest Rutherford’s work on radioactive elements won him the Nobel prize for chemistry in 1908. And more groundbreaking discoveries were to follow
It was whilst digging potatoes in his family’s garden at Pungarehu in New Zealand that the future Lord Rutherford of Nelson received the telegram that changed his life. On that fateful day in 1895 the 23 year old physicist learned that he had won a prestigious science scholarship, which would enable him to continue his postgraduate studies abroad. Throwing down his spade he exclaimed, ’that’s the last potato I’ll ever dig!’ Soon he was on his way to the UK, to study at Cambridge’s Cavendish laboratory and begin the research career which gained him a Nobel prize a century ago.
Ernest Rutherford was born on 30 August 1871, in a rural district near Nelson on New Zealand’s South Island. His father was, at various times, a wheelwright, farmer, logger, flax miller, bridge builder and general ’bush engineer’. His mother was a schoolteacher. Rutherford, a heavily built young man, played rugby with enthusiasm, and excelled academically. He won scholarships to continue studying past the normal school-leaving age, and, in 1889, entered Canterbury College in Christchurch where he gained BA, MA and BSc degrees. Even before graduating, he published original research on electromagnetic waves (recently discovered by Heinrich Hertz), which were soon to become the basis of radio communication.
By the time he left college, a career in physics was Rutherford’s goal. He realised it would be difficult to pursue so far away from major centres of research and his big opportunity came in that telegram - he was awarded a travelling science scholarship, funded by the profits of England’s 1851 Great Exhibition. Even with his early achievements, it was partly through luck that he received the offer at all - the young chemist who was the committee’s first choice decided to get married and stay in New Zealand.
Rutherford also had plans to marry. When he left New Zealand he was already unofficially engaged to Mary Newton, the daughter of his Canterbury landlady. But the couple agreed to delay their wedding until he could establish himself in the academic world. They finally married in 1900, and had one daughter, Eileen, born in 1901.
When Rutherford reached Cambridge in the autumn of 1895, his supervisor Joseph ’JJ’ Thomson encouraged him to continue working on the generation and detection of radio waves. He had already achieved some success with this when, towards the end of 1895, Wilhelm R?ntgen’s discovery of x-rays astonished the scientific world. Thomson and Rutherford immediately began investigating the new rays together, taking particular interest in their power to ionise gases. Later in 1896 Henri Becquerel announced the discovery of another form of ionising radiation, emitted by uranium-containing minerals, and in the following year Rutherford turned his attention to it. He quickly identified two different kinds of rays, alpha and beta. Alpha rays were more effective at ionisation, but beta rays had greater penetrating power. He also detected indications of a third type, which were named gamma rays by Paul Villard in 1900.
New alchemy
Unable to secure a permanent position at Cambridge before his scholarship ran out, in 1898 Rutherford accepted the post of junior physics professor at McGill University in Montreal. Once settled in Canada, he was quick to resume his research. Other elements, including thorium, had recently been shown to exhibit radioactivity, and during the summer of 1899 Rutherford began examining some puzzling anomalies in thorium’s radioactive output. They convinced him that it was releasing a previously unknown gas, or ’emanation’, which was itself highly radioactive. Later, he found that when this emanation was left standing it deposited what appeared to be yet another radioactive substance on the walls of the containing vessel.
In the autumn of 1901, Rutherford sought the assistance of Frederick Soddy, a young English chemist who had recently arrived at McGill. Soddy found that the emanation would not combine with any of the usual reagents, and in 1902 the two men concluded that it must be a new element belonging to the argon family. (The gas was officially named radon in 1924. The active deposit it left - which underwent several further stages of decay - was eventually identified as polonium).
Rutherford became convinced that when radiation was being emitted one element was actually changing into another, and Soddy’s chemical expertise helped to confirm this. They showed that the process of radioactive decay followed an orderly sequence from element to element, each one having a distinctive half-life during which 50 per cent of it would be transformed.
In 1903 Soddy returned to the UK and continued working on radiochemistry. By introducing the concept of an isotope, he solved the problem of fitting the ever-increasing number of radioactive species tidily into the periodic table of elements and gained his own Nobel award in 1921. Meanwhile, back in Canada, Rutherford made further progress. He had already shown that alpha rays could be diverted by powerful electrical and magnetic fields, indicating that they consisted of charged particles. In subsequent years, more delicate experiments revealed that alpha particles (whatever their source) had a mass nearly twice that of a hydrogen atom, and a positive charge of two units. Rutherford inferred from this that alpha particles were ionised helium atoms. He demonstrated this conclusively after moving back to the UK to become Langworthy professor of physics at Manchester University in 1907.
In 1908 Rutherford received the Nobel prize in chemistry for his work on the radioactive elements, after which several gifted researchers came to work under his supervision at Manchester. He was instrumental in persuading the government to direct more funding into science - and securing the resources that allowed him to take on many students as well as to carry out his own research.
Smashing the atom
In 1909 he directed Hans Geiger and Ernest Marsden to beam alpha particles at a sheet of gold foil and observe any change in their trajectory. Rutherford already knew that while most alpha-particles passed straight through such obstructions, some were slightly diverted. But to his astonishment, Geiger and Marsden found that a few alpha-particles changed direction quite radically. He remarked that it was as if an artillery shell fired at a sheet of tissue paper had bounced back.
Rutherford realised that any alpha particle deflected through such a wide angle must have bounced off another positively charged body. But, since most alpha particles passed straight through the foil, such collisions were clearly rare. He concluded that most of an atom’s mass was concentrated in a small positively charged nucleus, surrounded by a cloud of negatively charged electrons (beta particles) which occupied much more space. (This concept had been partially anticipated in 1904 by the Japanese physicist Hantaro Nagaoka, whose ’Saturnian’ atom had a ring of electrons around a central nucleus - though without experimental backing it gained little support.)
Rutherford announced his dynamic model of the nuclear atom early in 1911. It displaced J J Thompson’s static plum pudding atom, which had negative electrons embedded evenly throughout a sphere of positive electricity. But the new model raised problems of its own. According to the accepted laws of electrodynamics, the orbiting electrons should be radiating energy continuously while spiralling into the nucleus. However, in 1913 another of Rutherford’s younger collaborators, Niels Bohr, applied the new quantum theory of energy exchanges to this anomaly. He proposed that electrons could occupy certain specific orbits without loss of energy, only absorbing or emitting a fixed quantum of radiant energy when they jump to a higher or lower orbit. And Bohr’s equations delivered a crucial bonus - these orbital jumps could be correlated with lines in the emission and absorption spectra of the elements.
When war broke out in 1914 Rutherford became heavily involved in military and naval research, doing valuable work on acoustic methods of submarine detection. He also served on several government committees. But his research was unhampered by his military obligations. He still found time to perform the first artificially induced atomic disintegration, by bombarding nitrogen atoms with alpha-particles. It was no surprise when he was invited to follow Thomson as the Cavendish Professor at Cambridge in 1919. He remained there for the rest of his life, overseeing the laboratory’s expansion and teaching, advising and encouraging outstanding younger physicists including James Chadwick and Patrick Blackett, both of whom won their own Nobel prizes.
Rutherford was always an inspiring teacher, but his students recalled especially his kindness to scholars who - like his younger self - had arrived in Cambridge from abroad and knew nobody there. He also welcomed female students into his lab, and encouraged them to engage in research - an unusually liberal attitude in those days. In 1933 he became a leading member of the Academic Assistance Committee, which helped to relocate scholars fleeing from persecution in Nazi Germany. According to one biographer, Rutherford never lost a friend or made an enemy during his career - a remarkable achievement in Cambridge’s highly competitive academic environment.
By his untimely death in 1937, from complications following abdominal surgery, he had gained almost every major scientific honour. He was elected a fellow of the Royal Society in 1903, received its Rumford medal in 1904 and its Copely medal in 1922, and served as its president from 1925 to 1930. He was knighted in 1914, admitted to the Order of Merit in 1925, and became Baron Rutherford of Nelson in 1931. Universities and learned societies in many other countries presented him with honorary degrees and awards. Yet he always remained the same bluff and unpretentious New Zealander, who might interrupt conversation over High Table in Trinity College with a cry of ’anyone for the Marx Brothers?’ when one of the comedians’ films was featuring at a local cinema.
Rutherford was a physicist through and through - famous for his jocular remark that ’all science is either physics or stamp-collecting’, but his career exemplified the openness of the frontier between chemistry and physics, across which valuable transactions are continually taking place. His work on the radioactive elements made such a vital contribution to the progress of chemistry that the Swedish Royal Academy had no hesitation in awarding him the Nobel Prize for that subject, rather than for his own.
It is 50 years since Frederick Sanger was honoured with the first of his two chemistry Nobel prizes, for uncovering some of life’s key chemistry

The young Frederick Sanger was - as he later admitted - an ’above-average but not outstanding’ student who won no prizes. It was an apparently casual decision made soon after starting his degree programme that led him eventually to the greatest prize in science. He needed another half-course to complete his timetable, and despite knowing nothing about the subject, he chose biochemistry, simply because it sounded interesting.
The subject clearly exceeded his expectations, and, after graduation, he went into biochemical research. Although early studies included mundane but practical work on the nutritional content of potatoes, he soon moved to more fundamental research on the chemistry of life. This led him to a Nobel award shortly after his 40th birthday - and a second one two decades later. He is the only person ever to have received two Nobel prizes for chemistry.
Sanger was born on 3 August 1918 in Rendcombe, Gloucestershire, in the UK, where his father was a medical practitioner. His mother was the daughter of a cotton manufacturer. He was educated at Bryanston School in Dorset and St John’s College, Cambridge. After completing his science degree in 1939 he took an advanced course in biochemistry, and surprised himself and his teachers by gaining first class marks. In 1940 he decided to continue at Cambridge with his family supporting him financially until he gained a research fellowship. In the same year he married Margaret Howe. They had three children - Robin (born 1943) Peter (born 1946) and Sally Joan (born 1960).
Science through war
As a Quaker pacifist Sanger was exempted from war service, though some of his early work on foodstuffs did have strategic implications. For a short time, Sanger assisted Norman Pirie in his attempts to extract edible protein from grass, and he later worked with Albert Neuberger on the nutritive constituents of potatoes.
His major investigation, however, was into the metabolism of the amino acid lysine. After gaining his PhD in 1943, Sanger began working on proteins with Albert Chibnall, the university’s new biochemistry professor, who steered him towards insulin. Some scientists still believed that investigating proteins by chemical methods was a hopeless task, but several biochemists - Chibnall among them - were prepared to try.
Emil Fischer had already discovered that proteins consisted of chains of amino acids linked together by peptide bonds ( see box ) When Sanger started his research, biochemists had already succeeded in splitting some protein molecules into their constituent amino acids. No more than 20 standard types of amino acid occur in almost all known proteins, though there can be hundreds of amino acid molecules in a polypeptide chain. Sanger later recalled that the Cambridge group targeted insulin because of its medical importance - as a treatment for diabetes - and because it was available in pure form. It proved a fortunate choice; while some proteins have molecular weights running into millions, insulin’s - as Sanger discovered - is only 6000. It was still a huge and complicated molecule to tackle with chemical methods and confirming its formula took him and his collaborators over a decade.
Sanger developed a technique to fragment these protein chains. The fragments were then separated by chromatographic techniques, and their formulae established by further analysis. Using various reagents and enzymes to break the chains at different points, the entire insulin molecule was gradually mapped. In 1955 Sanger announced the first complete determination of the chemical formula for a protein, an achievement for which he received the 1958 Nobel prize for chemistry.
Piece by piece
After this triumph came what Sanger later described as ’the lean years’, when he tackled interesting problems but made no major discoveries. This period ended in 1962, when he joined the Medical Research Council’s Laboratory of Molecular Biology (LMB) in Cambridge. Among his colleagues were a cluster of future Nobel laureates including Max Perutz, Francis Crick and John Kendrew, all of whom earned their prizes in 1962. Also involved were Aaron Klug and Sydney Brenner, who were similarly honoured in 1982 and 2002 respectively. In such stimulating company, Sanger turned his attention from proteins to nucleic acids, joining the race to crack the genetic code.
Once again his target was a huge molecule composed of many subunits. While the protein alphabet consists of 20 amino acid letters, the nucleic acid alphabet has only four - but the sequences in which they are arranged are far longer. To explore them, Sanger assembled a gifted team, among whom Bart Barrell, Alan Coulson and George Brownlee were prominent.
RNA (ribonucleic acid) molecules are relatively short chains, some containing under 100 nucleotides. Each nucleotide consists of three parts - a sugar molecule (ribose in RNA), a phosphate group, and one of four organic bases (adenine, cytosine, guanine and uracil). The sugar and phosphate components link to form the molecule’s backbone, with one base attached to every sugar. This sequence of bases gives each RNA molecule its specific function within a living organism.
The new reagents and techniques Sanger developed to break nucleic acid chains were invaluable to break apart the unfathomably large structures of RNA molecules. His team applied them on a massive scale to separate and identify the fragments. One of his key innovations was using radioactive phosphorus atoms to track nucleotide fragments through complex chromatographic and electrophoretic separation processes. In 1965 Robert Holley achieved the first sequencing of an RNA molecule, containing 77 nucleotides. However, by the late 1960s Sanger’s group were applying his new techniques to sequence RNA molecules consisting of several hundred nucleotides.
They then applied the same techniques - and some new ones - to DNA (deoxyribonucleic acid). Its fundamental structure is similar to that of RNA, but with some key differences - the sugar is 2-deoxyribose rather than ribose, and one of the four bases is different (thymine instead of uracil). Most significantly, while RNA’s polynucleotide chains are single-stranded, DNA’s double chains are cross-linked by bonds between complementary base pairs, and twisted into a spiral - the iconographic double helix. DNA chains are also much longer, consisting of thousands, or even millions, of nucleotides which can specify in detail the make-up of a living creature.
In 1977 Sanger’s group was the first to determine the complete sequence of a DNA-based genome. It was a virus, the bacteriophage phiX174, which contains a relatively tiny 5386 nucleotide pairs. For this achievement, Sanger received the 1980 Nobel award for chemistry. It was shared with Paul Berg and Walter Gilbert, both of whom had also made outstanding advances in this area. Over the next five years, Sanger’s team streamlined his methods to crack the sequence of human mitochondrial DNA, which has around 16 000 nucleotide pairs, and bacteriophage lambda, which has over 48 000.
Sanger retired from full-time research in 1983, wishing to devote more time to his family and his hobbies (gardening and boating). Since then his investigative method - Sanger sequencing - has been employed to read DNA sequences up to three billion units long. During his career he has received many honours besides his Nobel awards. He was elected a Fellow of the Royal Society in 1954, awarded the CBE 1963, made a Companion of Honour in 1981, and admitted to the Order of Merit in 1986. In 1992, the Wellcome Trust and the Medical Research Council established a new research centre ’to further our knowledge of genomes, and in particular . the sequencing and interpretation of the human genome’, naming it the Sanger Centre (now the Sanger Institute) in his honour.
Sanger reached over the open border between chemistry and biology, developing new chemical techniques for investigating molecules which are the fundamental building blocks of life. Either of the discoveries which gained him Nobel awards for chemistry might have earned the equivalent prize for an outstanding contribution to physiology or medicine.
Mike Sutton is a senior lecturer in history at Northumbria University, UK
Further Reading
- D Wilson, Rutherford, Simple Genius , London, Hodder & Stoughton, 1983
- M Oliphant, Rutherford: recollections of the Cambridge days , Amsterdam, Elsevier, 1972
- E Rutherford, Nobel Lecture 1908: See Website
- E Davies, Chemistry World , December 2005, 44
- F Sanger,Annual Review of Biochemistry , 1988, 57 , 1
- F Sanger, Nobel Lecture 1958: See Website
Supersized molecules
Sanger’s success was in breaking down proteins - dauntingly large and complex molecules - and enabling the detailed study of their structures using chemical methods.
Breaking it down
When Sanger started his work on insulin, the basic chemistry of proteins had been established - it was understood that each protein contained a different number of amino acids. A typical amino acid has the formula: NH2CH(R)COOH (R represents an organic side chain which may contain oxygen, nitrogen or sulfur atoms as well as carbon and hydrogen). Two amino acid molecules can condense, forming a peptide bond, and large numbers of amino acids can combine to form polypeptide chains. For example: NH2CH(R)CONHCH(R’)CONHCH(R’’)CONH- etc. These polypeptides fold into the three-dimensional tangle of a protein, such as insulin (pictured). Sanger’s first step was to develop a method for identifying the amino acid at the end of a polypeptide chain. In 1945 he found that 1,2,4-fluorodinitrobenzene bonded with the NH2 group at the amino end of the chain, but not with the NH groups of the peptide links in the middle. When the peptide links were broken, the tagged amino acids could be separated from the mix chromatographically, and then identified. After much effort, this method revealed that the insulin molecule consisted of two polypeptide chains, one ending in glycine (NH2CH2COOH) the other in phenylalanine (NH2CH(CH2C6H5)COOH).
Manageable molecules
Sanger deduced that these chains were cross-linked by sulfur-sulfur bonds between adjacent molecules of cysteine (NH2CH(CH2SH)COOH). This was confirmed by breaking the sulfur links selectively with performic acid (HCOOOH) and isolating the two chains. One was found to consist of 21 amino acids, the other of 30. He then used new reagents to break the chains into smaller blocks, each containing only a few amino acids.






No comments yet