Turning an active drug molecule into a finished product requires as much chemistry as developing the drug in the first place, as Phillip Broadwith discovers
Turning an active drug molecule into a finished product requires as much chemistry as developing the drug in the first place, as Phillip Broadwith discovers

Discovering a new drug is only the first step in the development of a new medicine. Once a promising molecule is identified, it falls to the formulation scientists to work out whether it can be turned into a tablet or an injection, an inhaler or a topical cream, a single-shot dose or an extended-release pill. Then they have to work out how to create that formulation.
‘No matter what else you may think about, with all formulations you have the major goals of making the product physically and chemically stable, processable in manufacturing terms and bioavailable at its site of action,’ says Andrew Dennis, an associate director in the portfolio-enabling technologies group at Bristol-Myers Squibb (BMS).
Even for a relatively mundane, classical formulation tablet, this means considering all kinds of characteristics, as Vitaliy Khutoryanskiy of the University of Reading, UK, explains. ‘Active pharmaceutical ingredients [APIs] are typically delivered at low doses, so if you made tablets from just the active ingredient they would be too small – you’d need a pair of tweezers to handle them,’ he says. Typically, water-soluble sugars such as lactose would be used to bulk up APIs to an appropriate size.
‘But if you just used lactose and the API, then you wouldn’t be able to compress it into a tablet,’ Khutoryanskiy continues, ‘so you need to add another ingredient to make sure you compensate for that brittleness. Something elastic, like microcrystalline cellulose.’ Then a binder might be added to make sure the mixture forms granules that will flow easily through the processing machinery. Once the tablet has been pressed and packaged, it needs to survive on the shelf until it is swallowed. Then it needs to dissolve at the right time, ‘So you might add a polymer that will swell to help the tablet disintegrate,’ Khutoryanskiy adds.
A pill to change behaviour
Not all of the challenges a formulator faces are posed by chemistry. How a medicine is presented can have a huge effect on getting people to take their medication properly, as Robert Becker, chief research officer at specialist formulation company Aptalis explains. ‘It could be very simple things like size, or colour, or the organoleptic properties – for example smaller children and older people are unwilling or unable to swallow tablets.’
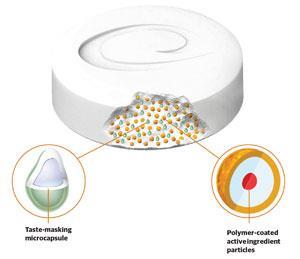
For this reason, as well as convenience for medicines that need to be taken several times a day, there is an increased demand for flavoured liquid formulations or oral disintegrating tablets (ODTs). These are placed on the tongue and quickly break down into smaller, more easily swallowed particles. But ODTs come with their own drawbacks. ‘The active ingredient gets in contact with the saliva,’ says Becker, ‘and for soluble APIs, you are exposed to the bad taste of the drug – many drugs that are water soluble taste bitter.’
To overcome this, the traditional approach would be to add a flavouring and/or sugar to mask the bitterness. But as Becker explains, this only works well in a minority of cases. ‘The best thing to do is to coat the active ingredient particles [within the ODT] with a polymer film to prevent dissolution,’ he says. ‘Then you swallow the coated particles, which dissolve quickly in the stomach due to the pH change. If you like, you can still add flavour or sugar to make it more pleasant, but you never come in contact with the API.’
Another way to help patients stick to their medication regimes is to switch from formulations that require multiple daily doses to a single dose that releases its drug load over an extended period or at a specific point in the digestive tract. While many companies have developed slow-release formulations, Aptalis’s approach is to use small particles coated in layers of different polymers that control how the drug is released.
‘The smaller the particles, the more they behave like a liquid,’ Becker says. Slow-release pills that do not break down into particles tend to get stuck – either in the stomach or in pockets in the gastrointestinal tract – so they cannot move along with the liquid and the food and cannot release their drug payload homogeneously.
‘We have developed specific combinations of polymers that enable us to release the API over different timed-release profiles,’ he adds. This can either be controlled by diffusion, or by exploiting different layers that are sensitive to pH changes in the digestive tract, such as moving from below pH2 in the stomach to pH5 in the small intestine or pH8 in the colon.
Balancing biologics
As well as more complex delivery systems, formulators are increasingly coming up against the challenges of creating medicines based on larger and more complex molecules, particularly as the number of biological drugs being approved increases. And while companies have been formulating live bacteria and viruses for vaccines for many years, the drive to spread vaccination programmes into developing countries that lack the necessary cold-storage infrastructure provides an opportunity to develop innovative ways to stabilise them.
‘Half the vaccines in the world are destroyed because they haven’t been stored properly,’ says Sam de Costa from Nova Laboratories, a UK formulation company. ‘What we’re trying to do is make products that can be stored at ambient temperature anywhere in the world.’
Nova has developed a way to store desiccated products protected by a mixture of amorphous, glassy sugars. It works by drying a mixture of the active ingredient and the sugar blend onto a filter paper-like membrane, which is then packaged in a plastic case with ports for a needle and syringe. As the liquid from the syringe floods the device, it reconstitutes the dried material and can then be injected directly into the patient.
The glassy, amorphous state of the sugars is crucial to the device’s success. ‘It’s almost a lesson from nature,’ says de Costa. ‘Certain animals and plants can survive desiccation, then the moment you get some rain they come back to life. When you get these harsh conditions, you get more trehalose accumulating in the intracellular space. It becomes like a syrup protecting the organelles – it inhibits molecular movement and reduces the water content and preserves biological activity.’
More than tablets
Khutoryanskiy’s group at Reading focuses mainly on delivering drugs across mucosal tissues including the eyes, nose and gums. He explains that the bioavailability of compounds administered as eye drops is very low – usually only 3–5% of the drug is absorbed. ‘People have tried intra-ocular injections,’ he says. ‘These are very efficient but very painful.’ Adding viscous polymers to eye drops or making mucoadhesive films that form gels on the surface of the eye can increase the drug retention quite dramatically, but often that is not enough.
The group is also looking at using chemistry to enhance the permeability of the cornea. ‘There is a condition called keratoconus, where the cornea is deformed and loses its mechanical properties,’ says Khutoryanskiy. ‘These patients gradually lose their vision.’ One treatment for keratoconus is to saturate the cornea with riboflavin and then irradiate it with UV light. This forms cross-links and stabilises the cornea structure. ‘But to saturate the cornea with riboflavin, you need to scrape off the epithelium. Imagine that the top surface layer of a patient’s eye is scraped off – it must be quite painful,’ he says.
The team has been working with cyclodextrins – ring-shaped sugar chains that can extract cholesterol from cell membranes. This changes the fluidity of the membrane and leaves behind tiny holes that let the drug through more easily. While they have had some success at improving penetration of riboflavin into the cornea, Khutoryanskiy notes that his approach would not be suitable for many other ocular medicines, as it does cause some damage to the cell membranes. However, as an alternative to surgical removal of the epithelium, it is promising.
There are many other mucosal surfaces in the body, and Khutoryanskiy has begun investigating drug delivery for bladder cancer. ‘The bladder walls are the most impermeable membranes in the body,’ he says, ‘but this opens an interesting opportunity.’ If the walls are very impermeable, then one could potentially deliver very high concentrations of cytotoxic anti-cancer drugs with a much lower risk of side-effects.
‘It’s a very difficult, very unpleasant way of delivering drugs, because they can only be delivered by catheter,’ he says. Then there is the problem that the drug will be constantly diluted with urine and expelled as the bladder is emptied. ‘At the moment we are looking at improving retention with mucoadhesive systems. We are testing with pig bladders to look at how different particles and polymers stick to the surface, and how they could be washed off by artificial urine.’
No magic ingredients
But if they are to be useful in the modern pharmaceutical industry, any new ingredients – developed either by academics such as Khutoryanskiy or companies like Aptalis and Nova – need to pass the stringent regulatory requirements set by bodies like the US Food and Drug Administration and the European Medicines Agency. The safety requirements for additional ingredients (also known as excipients) are just as strict as those for the active drug compound.
It is therefore quite difficult to introduce completely new materials into the market, and there is little incentive to do so. ‘Investing in a new excipient is a major decision,’ says BMS’s Dennis. ‘We would have to have a full package of toxicology and safety data to get it approved, so we would have to be convinced that it offered a significant benefit over the alternatives.’
So while there are a few new excipients being developed, companies are mostly investing in understanding and modelling the underlying chemistry of interactions between the extensive library of materials that are already available, and coming up with new combinations. And because of increasing regulatory demands on quality, formulators also need to know more about the chemistry or impurity profiles of existing excipient materials and how they are produced.
There’s a growing willingness between companies to work together at the fundamental science behind formulation
One of the biggest recent changes, Dennis says, has been a shift away from batchwise manufacturing processes towards continuous ones. ‘The benefit of a continuous process is that it makes scale-up easier, but also allows you to run more online monitoring of the performance and quality of the materials and the process,’ he adds.
One example is hot melt extrusion – a process originally employed in the plastics industry to shape polymers into sheets, tubes or fibres, which has gained rapid acceptance in pharmaceutical processing. John Jones, a senior scientist at BMS and committee member of the RSC’s Formulation Science and Technology Group, explains that the process allows drugs to be incorporated into a polymer matrix as very fine dispersions of a crystalline drug or as an amorphous solid solution. This allows the formulator to tune the dissolution rate and bioavailability of the drug, while keeping it stable on the shelf.
However, adapting the extrusion process for the pharmaceutical industry meant moving from single component polymer systems to often quite complex mixtures. ‘You need to consider interactions between the drug molecule and other excipients and the polymer,’ Jones says. ‘For example, you might need to add a shear flow-enabling polymer or even a surfactant.’
Looking to the future, Jones points to an emerging recognition that, at a basic level, formulation activities apply across different industries – for example petrochemicals or consumer products. ‘There’s a growing willingness between companies to work together at the fundamental chemistry and science behind formulation – to look at the processes they use and the expertise that they have and try to share that knowledge,’ he says. Dennis adds that the past regulatory constraints on the pharmaceutical industry could be seen as having limited innovation, but this kind of cross-industry collaboration initiative could bring in processes and expertise built up in the food and consumer products industries. ‘Combined with the changed regulatory environment, this should give us more opportunities to innovate and be more flexible, and see how we can catch up.’
Robocap
It’s all very well developing sophisticated sustained or delayed release formulations, but how do you know when or where in the gut it will be best to have your drug released? Getting that kind of pharmacokinetic information traditionally means preparing and testing a variety of different formulations. Jeff Shimizu from Dutch company Medimetrics explains that they have developed an electronically controlled capsule that can be programmed to accurately and flexibly release a formulation as it travels through the gut.
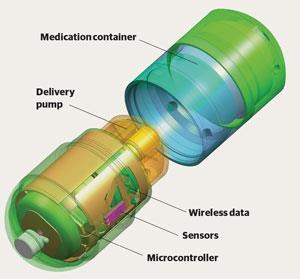
The IntelliCap device contains a microprocessor and an electric stepper motor, which pushes a solution, suspension or gel formulation out of a container on top and delivers it into the gut. It is also fitted with a pH sensor. ‘That tells you when the capsule moves from the stomach to the small intestine, then from the small intestine to the colon,’ says Shimizu. ‘Those are the two major landmarks you need to know when you’re distributing the drug in the gastrointestinal tract.’
Knowing exactly where in the gut and how fast the drug is released allows much better correlation with levels of drug in blood samples taken during trials. ‘Normally you would assume an average time,’ says Shimizu. ‘The published averages are pretty good, but there’s always variation, so knowing those time points for each individual is a real advantage.’
This gives formulators valuable information about where and how fast the drug is absorbed and metabolised, so that they can design a solid tablet or capsule formulation that will replicate the release profile from the intellicap.












No comments yet