Researchers in the US have discovered a new way to create the elusive discrete form of uranium nitride
Researchers in the US have discovered a new way to create the elusive discrete form of uranium nitride. The compound is important because its ceramic state, uranium mononitride, is a candidate for nuclear fuels of the future. However, little is known about the functionality of the uranium-nitrogen triple bond. The new work, by a team from Los Alamos National Laboratory in New Mexico, has shown that the nitride is surprisingly reactive and can activate C-H bonds in a way similar to an important enzyme in nature. The work could have implications for how spent uranium nitride fuels are stored and disposed of after they have been used.
Nitrides are a rare functional group for uranium and until now there have been no examples of a molecular terminal uranium nitride that could be studied. ’Molecular uranium nitride model compounds can help us better understand the functional properties, electronic structure, and chemical reactivity of a single isolated uranium nitride unit, really opening up a new chapter in uranium chemistry,’ says Jaqueline Kiplinger, who led the study.
Unlike the ceramic state of the compound, which contains multiple repeating units of uranium triple bonded to nitrogen, the nitride made by the Los Alamos researchers contains only one uranium nitride unit. The team made the compound by attaching a range of stabilising ligands to the uranium of uranium azide, U-N=N=N. When this precursor molecule was exposed to UV light, it photochemically loses a dinitrogen molecule to produce the nitride.
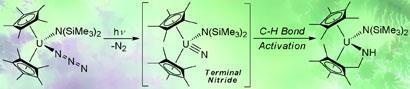
Very quickly, however, the uranium nitride reacts with a neighbouring C-H bond of one of the adjacent ligands.
’Interestingly, the observed carbon-hydrogen bond activation is strikingly similar to the oxidation of alkanes to alcohols by the enzyme cytochrome P450, which is required to oxidise organic substances in nearly all living organisms,’ says Kiplinger.
Kiplinger adds, ’If we are going to use uranium nitride as a fuel, our studies suggest that perhaps we should take a little closer look at it to understand better how it behaves. I think we are seeing uranium nitride in a new light.’
Polly Arnold, who researches small molecule activation by uranium complexes at Edinburgh University in the UK, is impressed by the study. ’It is a very, very nice piece of work,’ she says. ’To make a terminal nitride has been one of the holy grails of uranium chemistry. They show that their reaction must proceed via a terminal nitride, which then goes on to bite its own ligand.’
If the system could be modified to react with an externally introduced alkane, rather than one from its own ligand, it could represent a useful new way to activate C-H bonds in a controlled way, Arnold adds.
Simon Hadlington
References
10.1038/nchem.705






No comments yet