Gold surfaces polished atomically smooth by Fenton's reagent
Chemists from Germany and Poland have discovered a new way to polish gold completely smooth using Fenton’s reagent, producing gold surfaces that could be used in the electronics industry and electrochemical processes.
Fenton’s reagent is a solution of hydrogen peroxide and iron that generates potent hydroxyl radicals which attack the smallest bumps and ridges on the gold surface and leave it atomically smooth.
Highly polished gold is very useful in for electronic and biomedical applications, as it has valuable electrical and magnetic properties and is slow to corrode in the human body. But getting a perfect finish is tricky because pure gold metal is relatively soft.
’Trying to get a very smooth surface is rather like polishing butter - it tends to smear,’ says Stephen Fletcher, an electrochemist at the University of Loughborough, UK. Chemical processes that polish surfaces further have been developed, but these tend to corrode the surface.
In an unexpected discovery, Fenton’s reagent - which is commonly used to destroy organic matter in wastewater - was found to work as a clean and selective polish by removing any small protrusions on the surface. ’Our work was not at all aimed at finding a new polish for gold,’ says Fritz Scholz of the University of Greifswald in Germany who led the collaboration with the University of Warsaw in Poland. ’We were studying the interaction of hydroxyl radicals with compounds on electrode surfaces.’
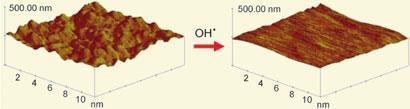
Scholz’s team observed that the hydroxyl radicals generated by Fenton’s reagent oxidise the gold surface in an unexpected way, dissolving off the highly reactive irregular bumps where the gold atoms are less ordered and leaving the gold surface smooth. The radicals leave a thin uniform layer of gold oxide against the smoothed surface, where the gold atoms are much more ordered.
Once the reaction is complete, the gold oxide layer can simply be reduced back into elemental gold. Since the process is a straightforward dip-and-dry method, Scholz’s team are confident that it can be applied to many shapes and sizes.
’The resulting gold surface is very mirror-like, and we are curious to see what applications the technique will find,’ Scholz says. A particular area of potential value is the medical industry - where implants could be pre-treated in this manner to minimise any atoms of gold that might escape into tissue.
’This technique may have important applications in the metal finishing industry because of its simplicity and the fact that it can be applied to objects of complex shape,’ Fletcher says. ’In addition, over the past several years there have been several dramatic advances toward the realisation of electronic computers integrated on the molecular scale. Smooth gold would provide an ideal contact for these devices.’
Lewis Brindley
References
et al, Angew. Chem. Int. Ed., 2010, DOI: 10.1002/anie.200906358






No comments yet