New breed of organocatalysts is set to improve on existing systems
Researchers in the UK have developed two new types of organocatalyst - catalysts made up only of organic building blocks. These new catalysts perform equally well, if not better, than existing organocatalysts in well-known asymmetric reactions. With only a small amount of the new catalysts needed to make the reactions work, they also prove easy to work with.
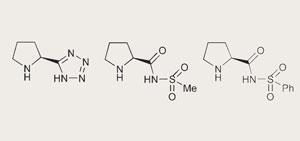
Steven Ley, at the University of Cambridge, began developing new catalysts to try to overcome some of the problems associated with other organocatalysts. Typical problems might be the organocatalyst’s limited solubility in different solvents, or the large amount of catalyst required to synthesise the product in a reasonable timescale. Organocatalysts are attractive because they offer a viable alternative to the sometimes toxic metal-based systems used.
Proline, a cyclic amino acid, is often used as an organocatalyst but reactions can be difficult due to problems with solubility. By adding hydrophobic groups to proline’s basic chiral backbone, the researchers showed that these new catalysts were also effective in non-polar solvents. Alexander Cobb, a post-doctoral researcher in the group, was particularly pleased with the performance of a catalyst with a tetrazole solubilising group. The loading - the amount of catalyst needed - was particularly low, justifying the five synthetic steps needed to make it from proline, he explained.
’The current work is a further development to find new and more effective catalysts,’ says Karl Anker J?rgensen, professor at Aarhus University, Denmark, adding that by ’combining the knowledge from this work, with the very extensive developments of new organocatalysts. new and more effective reactions can be performed.’
Vikki Allen
References
<man>b414742a</man>






No comments yet