Powerful new technique for precisely synthesising large molecules using simple templates
UK researchers have designed a new highly effective method to construct large molecules of a defined size using simple templates.
Recent approaches to the construction of nanomaterials have made use of advanced methods such as living polymerisation and self-assembly, but these techniques often produce a mixture of products. Taking inspiration from nature, which uses sophisticated templates such as the ribosome to make precise complex molecules, Harry Anderson’s group at the University of Oxford has developed a new strategy to synthesise macromolecules with precise lengths using basic templates.
’Up until now the group had been synthesising nano-rings of the same size as the template,’ says Melanie O’Sullivan, a lead author on the paper. ’When the synthesis of a 12-membered ring was taken up, we realised the difficulty of making a template of an equivalent size. To make larger rings we had to think of an alternative strategy.’
The new method makes use of a template with m number of binding sites and a building block with n number of binding sites. Under suitable reaction conditions, the template will direct the assembly of large molecules where the length of the molecule formed is the lowest common multiple of m and n.
This templating process is inspired by the Vernier scale, which is able to measure minute differences in size using relatively large objects. It uses the mismatch between the fixed and moving scale to achieve higher resolution.
The researchers synthesised a 12-porphyrin nano-ring, using a template with six binding sites and a building block with four binding sites. The method uses irreversible covalent bonding amongst the building blocks to drive the reaction to completion and thus achieves the desired products in high yields and fairly low reaction times.
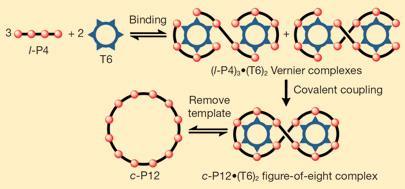
’This method, in principle, is extendable to much larger ring sizes,’ says researcher Harry Anderson. ’For example, only increasing the number of binding sites from four to five and still using the same template may increase the size of the ring from 12 to 30.’
With a diameter of 4.7nm, the 12-porphyrin ring is among the largest pi-conjugated macrocycles ever synthesised, and could be of particular interest as its photochemical and electrochemical properties resemble those of natural light-harvesting chlorophyll complexes.
’The strategy is remarkably efficient because it has an in-built error correction mechanism that collects the products of non-templated reactions that take place in solution,’ says Chris Hunter at the University of Sheffield, UK, a leading researcher in the development of functional large molecules. ’This work is certainly the most exciting development in porphyrin chemistry in many years.’
Akshat Rathi
References
M C O'Sullivan et alNature, 2011, DOI:10.1038/nature0968






No comments yet