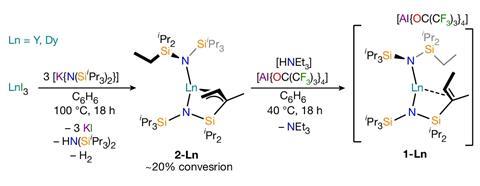
A new dysprosium complex retains magnetic memory at 100K, the highest temperature recorded for this class of compound. The researchers behind the work attribute the material’s properties to its unusual linear structure, and suggest that such complexes could drastically reduce the space required to store large amounts of information in modern data centres.
Single-molecule magnets can reduce the physical size of storage systems by increasing data density. But once they have been magnetised by an external magnetic field, these compounds generally need to be kept under extremely cold temperatures to prevent magnetic memory loss, limiting their everyday potential. One factor that has capped these compounds’ working temperatures to around 80K is the bent arrangement of bonds around their dysprosium atoms, which aids magnetic relaxation and loss of information.
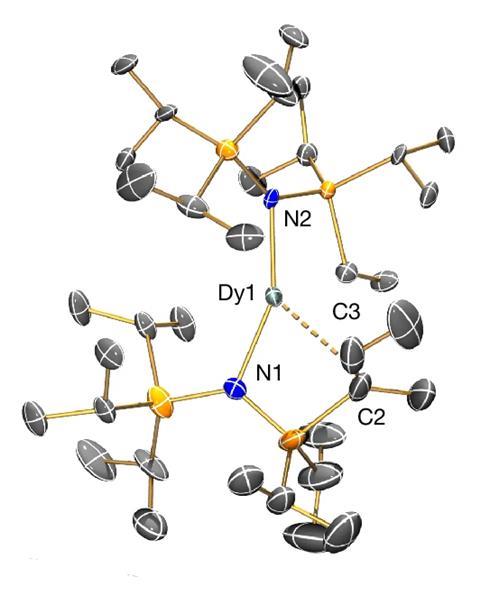
Now, researchers at the University of Manchester in the UK have synthesised a dysprosium complex in which the bonding around the central dysprosium atom takes on a more linear structure. An alkene incorporated into the ligand backbone helps pull the two dysprosium–nitrogen bonds into a straighter arrangement. This configuration increases the portion of angular momentum aligning with the principal axis, elevating overall magnetism of the compound. The linear motif also helps to slow magnetic relaxation, allowing the compound to remain magnetised up to 100K.
The researchers point out that because the magnet works at temperatures above that of liquid nitrogen (77K), its use could now be feasible in large data centres. The team now hopes to find even better single-molecule magnets by exploring complexes with even wider angles and more charge dense ligands.
References
J Emerson-King et al, Nature, 2025, DOI: 10.1038/s41586-025-09138-0
















No comments yet