Researchers in Switzerland have discovered how sulfonamide drugs - the first antimicrobial drugs to be discovered in the 1930s - cause the neurological side effects that sometimes occur following treatment. The finding improves our understanding of this class of drugs and might result in their improved use, the researchers say.
Sulfa drugs paved the way for the antibiotic revolution in medicine and have been in clinical use for over 70 years to treat many kinds of bacterial infections, including acne, chlamydia and pneumonia. They are also used to treat non-microbial conditions such as inflammatory bowel disease and rheumatoid arthritis. However, sometimes serious side effects can occur including allergic reactions and neurological disorders.
It was previously known that the anti-inflammatory drug sulfasalazine and its metabolite sulfapyridine inhibit the production of sepiapterin reductase SPR - an enzyme encoded in the human genome that is required for the biosynthesis of tetrahydrobiopterin (BH4). BH4 is critical for the production of neurotransmitters including serotonin and dopamine, and BH4 deficiency causes neurological problems similar to those associated with sulfonamide side effects.
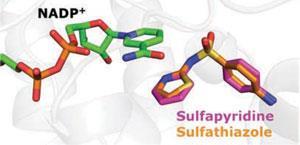
'That discoveries of this kind can still be made on such well-studied drugs is in our opinion very surprising,' says Johnsson. 'The finding that most members of this class of drugs interfere with BH4 biosynthesis helps us in understanding the pharmacology of these drugs and might result in their improved use.'
The team first obtained the crystal structures of SPR bound with a broad range of structurally diverse sulfa drugs which revealed how they achieve specific inhibition of the enzyme. Detailed cell culture experiments revealed that sulfa drugs inhibit BH4 biosynthesis and neurotransmitter production at physiological relevant concentrations.
'We then searched the literature for patient data that could be explained by an inhibition of BH4 and neurotransmitter biosynthesis and found a large number of clinical observations that could be rationalised by an inhibition of BH4 biosynthesis,' Johnsson explains.
'This work should provide a rationale to develop improved sulfa drugs with less neurological side effects,' comments Ernst Werner who investigates the biosynthesis and metabolic roles of BH4 at Innsbruck Medical University, Austria. 'For the scientist interested in tetrahydrobiopterin pathophysiology these novel findings are exciting since they identify approved drugs as potent inhibitors of tetrahydrobiopterin biosynthesis. This could provide a tool to test in humans hypotheses of benefits of inhibiting tetrahydrobiopterin biosynthesis, for example to ameliorate pain or limit cancer growth.
Johnsson acknowledges that more clinical studies need to be done to support their findings before improved therapies can be developed. 'There are approved sulfa drugs that do not inhibit SPR and these might be considered as replacements in cases where there are CNS side effects. However, this approach would need to be tested first through careful clinical studies. Also, we cannot rule out that some CNS side effects come from yet unknown interactions of sulfa drugs with other proteins.'






No comments yet