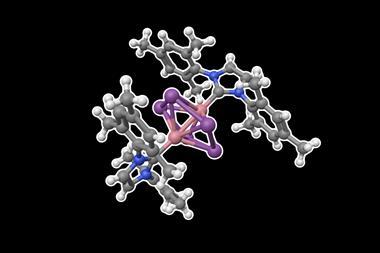
A new derivative of ferrocene has 20 valence electrons, challenging the longstanding rule that organometallic compounds can only have a maximum of 18. The researchers highlight that the ligand structure alters the redox properties of the complex, offering improvements for current catalysts and materials.
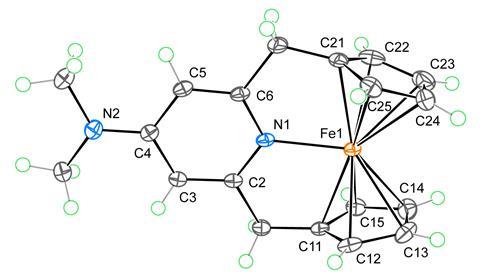
Organometallic compounds – species containing at least one metal-to-carbon bond – are often most stable if they have 18 valence electrons, leading to a rule that covers this. Chemists use this rule of thumb to rationalise a compound’s stability or predict a mechanistic pathway. Ferrocene – consisting of an iron atom sandwiched between two organic rings – is an energetically stable compound and the standard bearer for this rule. However, numerous examples break the rule, including the 20-electron nickelocene and certain square planar 16-electron complexes.
A collaborative effort between researchers in Germany, Japan and Russia has now led to the synthesis of ferrocene-based complexes with 20 valence electrons. ‘[This research] was curiosity driven,’ says Satoshi Takebayashi from the Okinawa Institute of Science and Technology Graduate University, Japan. ‘We just asked if [the 18-electron rule] could be breakable [with this type of complex].’
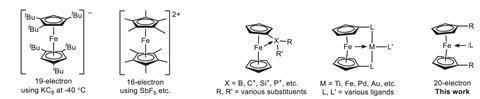
A tuneable ligand design allows for the reversible coordination of an intramolecular pyridine to the iron centre, increasing the electron count of the complex to 20. The researchers attribute this ability to the para-methoxy and para-amine substituents’ increasing electron density on the pyridine nitrogen, encouraging the formation of an iron–nitrogen bond. Computational studies reveal a decrease in covalent interaction between the iron centre and cyclopentadienyl rings to accommodate pyridine’s additional electrons.
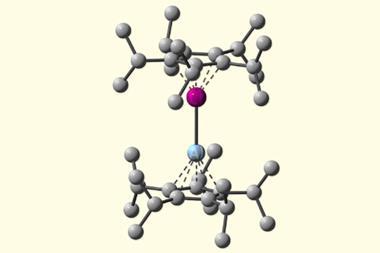
Further 18-electron complexes with the same ligand – including cationic cobaltocene and a neutral ruthenium sandwich complex – showed no bonding between the metal centre and pyridine nitrogen. Strong bonds between the metal centre and organic ring make the addition of two additional electrons unlikely. The scientists suggest that other neutral first-row transition metal complexes may be able to adopt a similar coordination to that of these newly derived ferrocene complexes.
Owing to their unique structure, the 20-electron ferrocene derivatives exhibit unusual redox chemistry. The compounds can reversibly transition under mild conditions between Fe(II), Fe(III) and Fe(IV). The partial occupation of high-energy antibonding orbitals enables the two-electron oxidation of these ferrocene derivatives. ‘I did not expect the second oxidation [to form Fe(IV)]. It was exciting, but it makes sense because we added two electrons to the system,’ Takebayashi says.
The team was able to use the relative ease of two-electron oxidation to create a dicationic species. ‘A ferrocene dication is normally really hard to [form] because you need highly oxidising conditions. And they can do it with this ligand set under quite mild conditions,’ notes David Mills, an inorganic chemist at the University of Manchester, UK.
Future research will explore the catalytic properties of these 20-electron ferrocene derivatives, with the hope that they may offer improvements for catalysts, medicines and advanced materials.
The researchers also hope to explore other unconventional compounds that defy standard chemical rules. Mills adds that ‘it’s nice to show people the exceptions, because I think that underpins why we use the 18-electron rule and why it generally works’.
References
S Takebayashi et al, Nat. Commun., 2025, DOI: 10.1038/s41467-025-61343-7












No comments yet