A mild and modular boron-mediated reaction sequence can now assemble diverse chemical building blocks into complex tetra-substituted alkenes – ‘a bit like Lego!’ one of its inventors says. The stereocontrolled process, which the team demonstrated in the synthesis of a panel of drug and natural product compounds, provides access to both E and Z isomers, depending on the chosen conditions. Subsequent computational studies revealed that this tunable selectivity arises from the formation of an unusual borenium intermediate, which the authors believe could have important mechanistic implications for other areas of boron chemistry.
Tetra-substituted alkenes – carbon–carbon double bonds surrounded by four different chemical groups – are a surprisingly common motif, found in natural products, drug compounds and materials chemistry. However, the intense steric crowding inherent in these structures means synthetic methods to reliably and selectively access such compounds are extremely limited.
Seeking a more modular solution to this problem, Varinder Aggarwal and his team at the University of Bristol harnessed the flexible chemistry of boron to break this assembly process into a sequence of controllable steps. ‘Boron is tri-coordinate. It has three groups attached to it, but it’s got a vacant orbital that allows it to accommodate a fourth group and become tetra-coordinate,’ he explains. ‘One can trigger what’s called 1,2-migration, where one of the groups on boron migrates to a neighbouring atom so it goes back to three groups.’
Inducing such a migration from a suitable alkynyl system would, therefore, generate a tri-substituted alkenyl boron, the team reasoned, with a subsequent step replacing the boron to form the final tetra-substituted alkene.
The sequence began by combining an alkylborane and an alkynyl lithium (R1 and R2) to produce the required alkynyl boronate intermediate. This was then exposed to an electrophile (R3), triggering the migration to an alkenylboron, which was stabilised in ester form. The team next investigated functionalising the boron (to install R4), reporting a variety of compatible reactions including Suzuki-Miyaura cross coupling, homologation and Zweifel olefination.
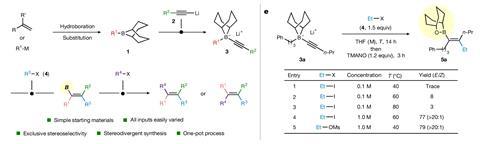
Intriguingly, the Zweifel reaction revealed unexpected stereodivergence: while the basic reaction returned the anticipated stereoinverted product, under neutral conditions, the alkene retained its initial configuration, enabling the group to access both E and Z products by tweaking the reaction conditions. Probing the mechanism behind this surprising observation, the group’s long-term collaborator, Rob Paton at Colorado State University, performed density functional theory studies to model the reaction under both basic and neutral conditions. ‘We found that the neutral reaction proceeds by a very interesting intermediate, which we termed a non-classical borenium ion – it has a three-centre, two-electron bond, and is a boron analogue of the non-classical carbocation,’ Paton explains. ‘It turned out to be a key species in understanding the unexpected stereoretentive outcome under neutral conditions.’
For Michael Greaney, an organic chemist at the University of Manchester, this tunable selectivity is the most important aspect of the work. ‘They’re tackling a really enduring problem – making tetra-substituted alkenes in a stereocontrolled way. That they can switch the selectivity from E to Z, it’s really impressive and a powerful approach,’ he says.
With four different modular building blocks and a mechanism to access either E or Z products, Aggarwal’s team demonstrated the reaction on an array of examples, including the anticancer agent tamoxifen and the insect pheromone γ-bisabolene. Moving forward, he hopes the reaction will be implemented by others in synthesis and the team are already investigating expanding the scope to include exocyclic and chiral substrates.
But the most significant impact of the work? ‘It’s that extra fundamental understanding – that’s one of the most exciting parts of it for me!’ Aggarwal says.
References
L Wei et al, Nature, 2025, DOI: 10.1038/s41586-025-09209-2













No comments yet