Chemists from the Swiss Federal Institute of Technology, ETH Zurich, have teamed chiral catalysts in pairs to selectively drive a reaction towards desired stereoisomeric products with high selectivity. Each catalyst activates one reagent and controls its substituent arrangement as it bonds to the other to form two neighbouring chiral centres. ‘We have shown that it is possible to develop fully stereodivergent reaction processes,’ says Erick Carreira, who led the work. ‘We expect that additional reactions displaying full stereodivergency will be identified.’
Catalysts that control one chiral centre are already well-known in organic chemistry, directing reactions to form predominantly one of two possible mirror-image enantiomeric products. Controlling configurations when forming more chiral centres is harder, and when it is possible the catalysts used typically only produce one possible diastereomeric configuration. Catalysts that interchangeably control each end of a newly-forming molecule are therefore attractive. ‘This concept has been out there as a “holy grail” of sorts,’ Carreira explains.
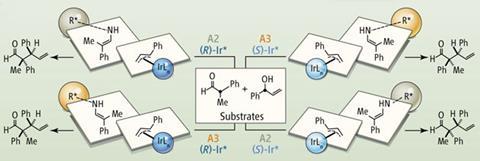
Iridium-catalysed allylation reactions have become useful for making chiral building blocks because nucleophiles add to the more substituted carbon. Carreira’s team has explored the reactivity of such iridium catalysts for almost 10 years. They realised that amine catalysts could be used to turn aldehydes into enamines that might work as nucleophiles in this reaction. They could then explore paired chiral catalysts – if they could find a suitable iridium complex. ‘The use of an allyl iridium complex such as ours that is compatible with amine catalysis was really key,’ Carreira said.
In the ETH Zurich scientists’ approach one of two iridium phosphoramidate catalyst enantiomers activates a secondary allyl alcohol. This reacts with an a-disubstituted aldehyde, activated by one of two amine pseudoenantiomers. Selecting catalysts controllably direct which of the four possible stereoisomers is the major product. They have explored a wide structural range, seeing many yields above 70%, with over 20-fold excesses of the desired diastereomer, in over 99% enantiomeric excess.
Those selectivities are ‘striking’ according to Harvard University’s Eric Jacobsen, who has developed dual catalysis techniques that can control a single chiral centre. ‘To have different catalysts activating each substrate is a beautifully simple solution to a long-standing problem,’ he tells Chemistry World. ‘The open question is: “Will this strategy be applicable to other reactions used in organic chemistry?” That remains to be seen, but a lot of people will be thinking about it now, with this result in hand, trying to find out.’






No comments yet