
Although elemental sulfur is noted for its high electrochemical capacity and refractive index, copolymerising it into a useful, workable material has met with little success. ‘Sulfur exhibits limited solubility or miscibility in the vast majority of conventional organic substances,' explains Jeffrey Pyun, University of Arizona, US, part of the team behind the new work. ‘To circumvent this challenge, we chose to conduct this new chemistry entirely in liquid sulfur as both the solvent and the reactive medium.’
Elemental sulfur primarily exists as eight-membered rings that, when melted, open to form chains whose diradical ends link up into linear sulfur polymers. But these polysulfanes are often chemically unstable and readily depolymerise back to the monomer rings. Using liquid molten sulfur as both the solvent and reactive medium however, the team could directly copolymerise the free-radical chains with the divinyl monomer 1,3-diisopropenylbenzene (DIB) without the need for additional initiators or organic solvents.
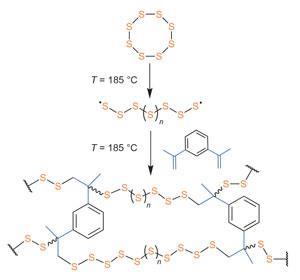
Pyun believes this kind of simple, but useful, chemistry will have a huge commercial impact, not least in the development of next generation lithium-sulfur batteries. One potential advocate is David Ainsworth, chief technical officer at Oxis Energy, UK, where pioneering work is currently ongoing on Li–S battery technology for electric vehicles, defence systems and portable electronic devices. ‘The initial cell cycling results look promising,’ he says. ‘It is very interesting as it represents a unique way of thinking with respect to sulfur based cathodes.’






No comments yet