A new reaction involving electron transfer from a highly electronically excited chemical species challenges a longstanding photophysical principle. By breaking ‘Kasha’s rule’, the transformation allows for new chemistry that would not otherwise be possible, according to the Swiss-based researchers who discovered it.
Kasha’s rule was first proposed by the physical chemist Michael Kasha in 1950, and states that reactions of a photoexcited molecule will only occur from its lowest electronically excited state due to the fast relaxation of higher-energy states.
Transitions from higher-energy excited states, known as anti-Kasha transitions, can occur in some cases involving a small number of molecules with unusually large energy gaps between their excited states, such as certain azulenes and zinc porphyrins. However, scientists have never previously observed such anti-Kasha reactivity in solution, as solvent molecules rapidly quench excited states.
Now, Björn Pfund and Oliver Wenger working at the University of Basel have spectroscopically observed anti-Kasha reactivity when reducing halogenated aromatic compounds.
The team first use a laser to excite a photosensitive molecule called dicyano-p-terphenyl (DCT), generating a radical anion catalyst. The DCT radical strongly interacts with a halogenated aromatic substrate, in a process known as pre-association. A second pulse of light a few microseconds later further excites the catalyst to a higher energy level, triggering an electron transfer to the aromatic compound.
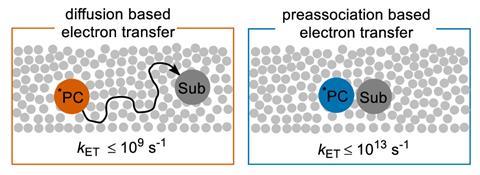
‘[The DCT anion] is so close in proximity [to the aromatic molecule] that the electron transfer is fast and competes with non-radiative decay to the ground state,’ says Pfund. The researchers observe an electron transfer rate on the order of 1013 s-1, several orders faster than the reaction rates seen in other reactions between a photocatalyst and substrate molecule.
Testing a wide variety of halogenated benzene compounds revealed that anti-Kasha reactivity requires a driving force of at least 1.2eV for electron transfer to occur. ‘These short-lived excited states require even higher driving forces than your average photoredox catalyst,’ says Bryan Kudisch, a photochemist at Florida State University in the US. ‘This knowledge has made me think about how to design other catalysts capable of anti-Kasha reactivity.’
Pfund, who has recently moved to Michigan State University in the US, says that the study ‘was not necessarily wanting to achieve a new kind of reaction, but to show that these higher excited states can indeed react’. He adds that other scientists might be able to use the new mechanism to develop future photocatalysts that are more selective or capable of catalysing difficult reactions, such as degradation of per- or poly-fluoroalkyl substances.
References
B Pfund and O Wenger, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c06115















No comments yet