A new light-driven molecular motor winds together hydrocarbon strands to make catenanes, without the need for templating. The system offers a way to make interlocked structures from starting materials that are incompatible with current methods.
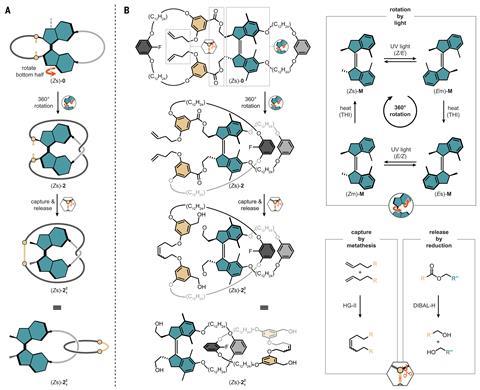
Although selective nanomachinery is ubiquitous in nature, designing molecular machines that use directional motion to control three-dimensional molecular shape is a major challenge. But now, Michael Kathan from Humboldt University of Berlin in Germany and his coworkers have harnessed a molecular motor’s mechanical rotation to produce entangled structures that would otherwise be thermodynamically impossible. The system eliminates the need for preorganised starting materials and templated reactions that are standard features in the synthesis of catenanes.
‘A new realm of chemical synthesis has been entered, which uses mechanical motion instead of chemical reactivity. This represents the emergence of an entirely new field of chemical research, connecting mechanical nanoscale motions with chemical structure formation,’ highlights Henry Dube, an expert in photochemistry and molecular machines from Friedrich-Alexander University of Erlangen-Nuremberg, Germany, ‘With this method, now it is possible to manufacture virtually any interlocked structure regardless of other functional groups being present or absent.’
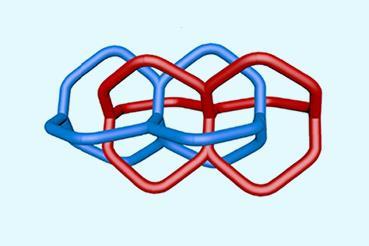
The motor developed by Kathan’s team has a double-bond axel that rotates in a single direction when light shines on it. Upon irradiation, photochemical double-bond isomerisation followed by thermal helix inversion results in a 180° rotational cycle. The process can be repeated, resulting in a full 360° turn. When two macrocycles are bound to the motor, each 180° rotation induces a crossing, winding the macrocycles into a helix. After the full turn is achieved, an olefin metathesis reaction closes the ring structure and the resulting catenane is released by cleavage of two ester-based anchors.
‘At the heart of our system is a light-driven molecular motor core that offers excellent selectivity during the winding process and, being a derivative of first-generation Feringa motors, provided sufficient kinetic stability to suppress unwanted thermal unwinding,’ explains Tommy Wachsmuth, who worked on the project.
The system offers a potential way to make catenanes from compounds that are incompatible with current templating strategies, such as commodity polymers and biomacromolecules.
According to Kelong Zhu, an expert in molecular machines and interlocked structures from Sun Yat-Sen University in China, the work ‘opens new avenues in molecular nanotechnology’. ‘The approach provides precise and selective topological control,’ he adds. ‘Each 180° motor rotation introduces a defined number of strand crossings, allowing for the fine-tuned assembly of interlocked structures.’
‘Conceptually, it reminds me of the powered stitching motions by a sewing machine – but at the molecular level,’ comments Ho Yu Au-Yeung, an expert in catenane chemistry from the University of Hong Kong. ‘By using a molecular machine that can be powered by external energy source, synthesis of mechanically interlocked molecules could be more efficient, which is essential for exploring their applications.’
Kathan explains that as well as extending the technology to knots and rotaxanes, the team now hopes to improve the efficiency and scalability of the approach. ‘A key challenge will be ensuring that the molecular motor can be removed from the interlocked structure and reused efficiently,’ he notes. ‘My personal goal is to reach a stage where the final mechanically interlocked structure gives no indication of how the interlocking was achieved – a kind of molecular craftsmanship where the process leaves no trace.’
References
T Wachsmuth et al, Science, 2025, DOI: 10.1126/science.adx536












No comments yet