From the discovery of aniline dyes to modern biofuels, chemistry and energy have always been intractably linked.
From the discovery of aniline dyes to modern biofuels, chemistry and energy have always been intractably linked.
Last year saw the 150th anniversary of William Perkin’s serendipitous discovery of mauveine, the dye that helped spawn the modern organic chemicals industry. Perkin isolated the purple compound while attempting to make quinine from aniline, a molecule found in the coal tar by-product of gas manufacture. Yet without the proliferation of gas lighting in Britain in the nineteenth century, there could easily have been no readily-available aniline, and no mauveine.

Chemistry’s historic links to energy production are frequently overlooked, even though the chemical industry’s raw material hydrocarbons are still provided almost exclusively by the same sources that power the lights and drive the wheels. The coal mines may have given way to oil wells, and the gasifiers may have been bypassed by natural gas pipelines, but the link between chemistry and energy remains intact. Indeed, even before the coal tar industry was founded in the UK, Britain was producing chemicals based upon the prevailing energy source of the time.
’It’s often forgotten that the first organic feedstock was wood,’ explains Peter Morris, an expert on the history of the chemical industry at London’s Science Museum. ’Wood was important as a source for methanol, acetic acid and also turpentine, which was used in the manufacture of Charles McIntosh’s waterproof coats. Wood remained important right up to the 1930s for chemical production, and also as an energy source.’ Ever since those early days of organic chemistry, chemists have explored the composition and manipulation of hydrocarbons, learning about their properties for combustion and finding new uses for seemingly worthless waste products.
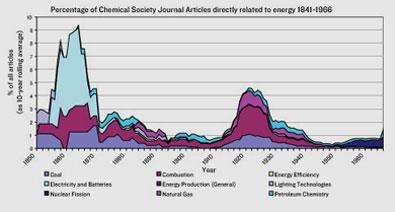
I wanted to test my suspicion that solving energy problems was nothing new for chemists. Not only does energy production shape the world they work in, it also directly dictates their own industry’s raw material supply. So how has energy shaped the development of chemistry? And with sustainable energy technologies on the rise, what does that mean for chemistry’s future? As I discovered, one need look no further than the publications of the British chemical societies for evidence.
A historic survey
A survey of the general-interest chemistry journals - from Proceedings of the Chemical Society of London (1842-1849) to Chemistry World (2004-) - revealed the responsiveness of chemists to energy issues. The trends that emerged from my survey of about 40,000 articles and meeting reports spread across 160 years of scholarly chemistry publications were fairly clear.
Between 1860 and 1870, almost 10 per cent of what was discussed by the Chemical Society was related to the introduction of electricity and its associated technologies, such as batteries. The dominant energy system was changing from coal and coal-gas to electricity, and the nation’s chemical scientists were at the forefront of the impending revolution. Electrical lighting was not actually commonplace on British streets until the 1890s. Yet ever since Michael Faraday’s Experimental Researches in Electricity was announced through their journal, the members of the Chemical Society appreciated their own importance to the introduction of electrical lighting, and also the application of electricity to chemical processes such as electrolysis.
In the hundred years after the 1890s we see two other periods when energy concerns took a more central role in chemical science articles: the 1920s and the 1970s. Both appear to correspond to major events in the history of energy provision.
The 1920s was the decade of the automobile. The availability of affordable cars coincided with the opening up of federal lands in the US to mineral exploitation, and an oil surplus. The increased use of abundant oil for transportation brought petroleum resources, including natural gas, into the energy equation in a big way. Petroleum was easy to extract, easy to transport and the by-products of gasoline production provided an excellent alternative to coal as a chemical feedstock. But despite this, the British Journal of the Chemical Society published more articles than ever before on coal.
This may reflect the fact that coal was so firmly established as the British chemical industry’s primary feedstock. And after the industry’s expansion during World War I, UK coal users needed to up their game in the face of competition from oil. The British government also had an interest in promoting the Empire’s coal reserves, as it had limited oil to meet the changing energy demands of warfare. This climate drove chemists, ever-responsive to the changing energy scene, to seek new uses for coal.
A similar effect is seen when looking at issues of Chemistry in Britain between 1965 and 2000. The great increase in articles on energy in 1975 compared to 1965 is almost certainly related to the 1973 oil crisis, when security of oil supply was threatened. The articles themselves were not concerned with oil, however - instead, renewables and energy efficiency dominated the chemical zeitgeist. With titles such as: Fermentation - An answer to the solvent crisis?, and Coal as a source of raw materials for the future, the potential impacts of insecure oil supplies on a discipline reliant on oil was not lost on members of the Royal Institute of Chemistry and the Chemical Society. Equally, conferences on topics including ’The Electrolytic Production of Hydrogen’ and ’The Hydrogen Energy Economy’ demonstrate the engagement of chemists with more general energy issues.
Shared history
The survey suggests that chemical scientists do indeed respond to changes in the prevailing energy system by actively focusing their research on energy, as both a feedstock consumer and technology enabler.
According to Peter Morris, an interesting example of this role for chemists is the story of the chemurgy movement in 1930s America. The movement promoted use of agricultural raw materials for industrial uses and was supported by Henry Ford, Thomas Edison and Irenee DuPont as a route towards economic independence. In the days before Middle East oil discoveries it was also endorsed as a replacement for declining US petroleum supplies. Agricultural crops soon became seen as a source of motor fuel and chemists developed ’agrol’, an ethanol/gasoline mix, which was marketed alongside plastics derived from soybean oil. Grain-derived ethanol was also a feedstock for synthetic rubber and it played a vital role in America’s World War II effort when rubber supplies were cut off. It was ultimately displaced by petrochemical rubber, just as petroleum became the major source of ethanol, but as Peter Morris points out, ’things could have gone in a quite different direction had the Farm Chemurgic Council been granted the contract for all US synthetic rubber at a time when polyethylene was also manufactured from alcohol rather than petroleum.’
Since 2004, the interest in solar photovoltaics, biofuels and hydrogen power amongst the pages of Chemistry World suggests that such concerns are once again high on chemists’ agendas. Novel techniques are constantly promoted for harnessing renewable resources; the availability of the dominant resource, oil, is once more uncertain. If we really are serious about accelerating the use of alternative energy sources, can the shared history between energy and chemistry point towards the best role for chemical scientists?
History tells us that we can reasonably expect chemists and chemical engineers to respond to changing energy systems. But the relationship works both ways, because the energy economy relies on the chemical sciences to utilise the by-products of fuel production as feedstocks. Chemical production and energy are mutually dependent. This suggests that an integrated approach to the two sectors has implications for how they might evolve together.
The carbon landscape
In the EU, 10 per cent of oil consumption is for chemical feedstocks. Global polymer production is projected to double by 2025, and the picture is similar for other chemicals. The carbon content for polymers alone would equate to roughly 63 million barrels of oil per year in 2025, twice the current UK consumption of motor fuels. According to BP, the current rate of consumption would exhaust known oil reserves in about 40 years, and any increases in demand for synthetic materials could reduce this figure regardless of advances in fuel efficiencies.
This underlines the importance of considering the entire hydrocarbon system and the potential for improvements in all areas. Even if they do not directly reduce carbon dioxide emissions, replacing oil feedstock with bioresources, using coal gasification, or investigating chemicals from carbon dioxide, could facilitate the technologies or economies of sustainable energy use.
Traditionally the energy and chemicals industries have been treated separately by governments with departments for energy detached from those for other industries. But that may be changing. In December 2006, the International Energy Agency held a workshop with members of the petrochemical industry to discuss feedstock substitutes, energy efficient technology and CO2 reduction as a part of the G8 Dialogue on climate change, clean energy and sustainable development. This was the first time the chemical industry had been explicitly invited to the IEA, and it may signal an understanding that the flows of energy products throughout the whole economy will need to be addressed for the substitution of oil to be as painless as possible.
Old ties, new directions
There is compelling evidence that 150 years of co-evolution of the chemistry and energy disciplines has created links between them than run deeper than simply the scientific contribution of chemists to improving energy technologies. As energy systems have evolved, so has the use of chemicals to produce dyes, synthetic materials and pharmaceuticals. Changes in feedstock demand have also been motivated by politics and tradition, as was the case with the slow transition to petroleum feedstocks in Europe compared to the US. Political commitment to local coal reserves ensured that coal chemistry still played a major role in Germany until the 1960s. When the shift was made it was to a light gasoline feedstock, as the European motor industry had come to use diesel fuel, leaving a gasoline surplus that was not present in the US where the chemical industry mainly consumed natural gas.
By considering the chemical and energy sectors as one evolving system, new opportunities for green chemistry are emerging that take advantage of the changing carbon landscape. Rapid expansion of biodiesel production to meet renewable energy targets has led to a glut of its by-product glycerol, with a resultant depression of the glycerol price. Combined with projected increases in biodiesel production, this has created opportunities to use glycerol in new ways that are now being recognised by entrepreneurs.
UK-based Davy Process Technology has developed a method for producing propylene glycol from glycerol. Vice-president Roger Lawrence says that provided biodiesel production is sustainable, bio-based propylene glycol could compete with the petrochemical-derived compound. ’But as long as the production costs of renewable fuels are higher than conventional sources, then government support through fiscal policy is essential. The danger is that projects or schemes that rely upon this are vulnerable.’ These risks can be managed by looking at a variety of renewable hydrocarbon sources, and Davy Process Technology have also developed processes to convert palm kernel oils to natural detergent alcohols, and bio-ethanol to ethyl acetate. Indeed, using by-product glycerol for chemical products echoes the use of residual petroleum fractions to launch the fledgling petrochemical industry.
Another example is found in Sweden’s ?rnsk?ldsvik region, which has a long and distinguished history in the conversion of forest materials to chemicals such as ethanol and acetic acid, alongside core pulp and paper activities. The region has responded to the explosion in global ethanol demand with the Processum biorefinery initiative, which aims to create a world-leading cluster of companies that will convert pulp and paper waste into fuels, packaging, pharmaceuticals, food and building materials.
Back to black
The resurgence of coal is also having an impact in many regions. As a more abundant fossil fuel than oil, and with a particular strategic importance in China and India (see Chemistry World: China, September 2007), use of coal is set to continue growing despite environmental concerns. To achieve higher efficiency in coal power stations and reduce greenhouse gas emissions, new combustion and carbon storage processes are in development (see ’How to bury the problem’).
The gasification of coal, however, has a rich history in the production of chemicals from syngas. In South Africa, Sasol has been producing fuels and chemicals from coal since the 1950s to avoid dependence on foreign oil.
Sasol has been perfecting the Fischer-Tropsch technique for half a century and now produces around 200 chemical products and fuels from over seven million tonnes of coal-derived Fischer-Tropsch hydrocarbons per year. In the current energy climate, Fischer-Tropsch projects such as Sasol and Qatar Petroleum’s gas-to-liquids venture are looking increasingly economically attractive. Sasol’s Johann van Rheede explains that ’a well thought through chemical co-production strategy will add value in the right circumstances. Our plants in Secunda demonstrate that the co-production of fuel and chemicals can be a viable business model. More development work needs to be done to address both technical and business challenges, but it can be anticipated that syngas-derived chemicals will gain market share as the GTL and CTL (coal-to-liquids) industry expands.’
The modern petrochemical industry exerts a powerful presence on the economy. But some major shifts are underway in both the energy and chemicals sectors that could have unpredictable impacts on the global hydrocarbon supply. Bio-based propylene glycol, integrated biorefineries and syngas plants all hold the potential to compete with their petrochemical counterparts if large scale production is achieved. The enduring link between hydrocarbon usage for chemicals and fuels that has so interested chemists for 150 years may be about to play an important role in the development of life after oil.
Simon Bennett is a researcher at the Centre for Energy Policy and Technology, Imperial College London, UK






No comments yet