Harry Gray has recently been awarded the Wolf prize for chemistry in recognition of his contributions to bioinorganic chemistry. Ian Farrell catches up with one of the most colourful characters in chemistry.
It was a fascination with coloured compounds that first attracted Harry Gray to chemistry. As a curious child his first (rather crude) experiments involved pouring fuming sulfuric acid over different coloured substances just to see what would happen.
’I mixed a few chemicals and made a few things, colours and stuff’, he recalls. ’I was fascinated with concentrated fuming sulphuric acid. I managed to find a supply house in Chicago that would sell me a big bottle of it. They didn’t know I was only 11 years old at the time. I’d send them an order letter and they’d deliver it to me. Looking back it was very dangerous - I poured it on all kinds of things and of course it mostly destroyed them; clothes of all kinds would turn totally black and charred.’
He talks with enthusiasm about the beginnings of a scientific curiosity that has changed very little over the course of his career. ’And explosions - a lot of kids will tell you they like explosions, and I did too, but what really fascinated me were colours. I had all kinds of chemicals that I acquired from various places, but it was the ones which were coloured which fascinated me the most. I wondered why cobalt compounds were sometimes blue and sometimes pink; why copper and nickel solutions were green; and I was fascinated by rubies, which were sometimes lightly coloured and other times dark, deep red - beautiful.’
At this stage, though, all these observations were without explanation: ’I could see some of these formulas in books and I had no idea what it was all about. But I had to figure out what these colours were due to. What made things different colours. Why were they so beautiful?’
In search of some answers Gray continued experimenting, graduating from Western Kentucky University in the US in 1957 with a BS degree in chemistry before working towards his PhD with Fred Basolo at Northwestern University. And his timing couldn’t have been better: the 1960s saw the gradual acceptance of molecular-orbital theory by chemists and the replacement of the less elegant valance-bond theory of molecular structure. Here was the tool he would need to answer his questions of colour: ligand field theory.
A postdoctoral position in Copenhagen, Denmark, preceded Gray’s first faculty position at Columbia University back in the US. ’I was writing lots of papers about electronic spectra and transition metal complexes and explaining a lot of the colour in great detail. This gave me a lot of satisfaction’. A reflection of the importance of this early work was Gray’s move to the prestigious California Institute of Technology (Caltech) in the US in 1966, where he has been ever since.
Changing colours
The move into bioinorganic chemistry came after comments from colleagues at Rockefeller University, Columbia and at Caltech. ’When you put a metal ion inside a protein, the colours are different from simple inorganic species. Many copper proteins have deep blue colours, not the kind of light blue you might associate with the copper sulfate you can get at the pharmacy. They have these deep-blue colours. In fact one of my favourites is the protein Azurin, which gives us the name for "Azure blue". I was fascinated with this, frankly - why is copper in a protein so much bluer? More intensely blue than just ordinary inorganic copper. And so I went into that area. In the late 1960s I felt I had to figure out these colours. What is it about a protein that makes these colours different?’
Looking for help, Gray turned to Robert Williams at Oxford University, UK, who had also been considering exactly this point. He had written that metal ions in proteins are in very special environments that don’t occur in simple compounds. Proteins are different because of the folded nature of the amino-acid chain.
Gray explains: ’I think what we did was to embrace Bob’s [Williams] ideas and Malstr?m’s [renowned biochemist from Gothenburg, Sweden] ideas and took them to the next level by figuring out exactly what these structures were. In the case of copper it was the protein protecting a sulphur, a thiolate sulphur deep inside the molecule, making it bind to the copper in a certain way. This copper-thiolate charge-transfer complex gives us the deep-blue colour. It gave me a great deal of satisfaction to figure that out. Out in the air, if you mix thiolate and copper you’ll get redox. You’ll get reaction chemistry: production of copper (i) and disulfide. But in the protected environment of a folded protein you can have copper(ii) and a thiolate together in a beautiful charge-transfer complex that gives this beautiful deep blue.’
Gray’s group worked on many proteins and successfully related their often unique colours to their structure. Copper, iron, and cobalt all got the same treatment. And then, in the early 1970s a student suggested the next move.
’His name was Bob Holwerder. I’d been fixated with colour and explaining it all my life and he looks at me and says "Harry, why don’t you do something important? Stop worrying about all these colours and spectroscopy. Why don’t we figure out how electrons move through proteins. Now that’s an important topic: that’s what runs life. It’s what keeps us alive. Electrons and respiration. Why don’t you figure that out?". So I said "OK, let’s work on it".’
They started this arduous task by studying the reactions of proteins with simple inorganic complexes of metals like chrome and iron. The breakthrough came in 1982. ’ We were able to demonstrate very distant chemistry - processes that took place over much longer distances than anyone had previously imagined. We found that you could transfer an electron between an iron and a ruthenium in a modified protein over a distance of 2nm. And it was fast. Very, very fast.’ What Gray had demonstrated is now known as electron tunnelling.
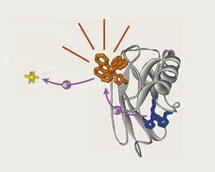
’Many people had speculated on it. Britton Chance [at the University of Pennsylvania, US] had hypothesised it was a mechanism that the body could use in respiration to keep us from burning up. And he was right - if it weren’t for electron tunnelling we would burn up. We eat strong reductants (fuel) and breathe in strong oxidants (air); in an automobile engine that would cause an explosion, so why don’t we explode? Why don’t we overheat? It’s because these redox units are orchestrated to be far apart. And there are guidance systems built in between them so that the electrons are carefully controlled throughout the network and the free-energy changes in each step are kept very small. At certain steps the energy is transformed to fuel, to ATP, rather than heat. By separating the components and then controlling the free energies of the tunnelling we don’t burn up and we don’t age rapidly. If this wasn’t the case, and you breathed in oxygen, all you’d be making is peroxides and oxy-radicals and you’d peroxidise all of your membranes. You’d age a lifetime in one or two days. Either that or you would explode! Instead we lead a reasonable life - in a situation where we have fuel and strong oxidants. It’s all managed by electron tunnelling’.
Business sense
That Gray is a successful scientist cannot be disputed, but what many don’t realise is that he is also a sharp business man. In 1989 Gray founded the Beckman Institute at Caltech, a new approach to multidisciplinary research which aimed to develop new technologies and instrumentation. ’In the early 1980s I had a feeling that chemistry and biology had to make a fundamental shift in infrastructure, moving from individual, single investigator labs towards shared instrumentation and technology. Bigger science, the way physics and astronomy have been going for some time.’
So Gray approached the infamous electrochemist Arnold Beckman, who had enjoyed many previous collaborations with Caltech, with several Beckman buildings already existing on campus. ’Beckman was interested in what we were doing, so we proposed to him the idea of an institute which would really be developing new instruments and new technology that would support basic research and the regular divisions of Caltech. The mission would be to develop new instruments which could be used by anyone.
’Dr Beckman bought into that. He made an initial $40m grant if we could match it with $10m. And another $10m if we could match that with another $10m, which we did. And there’s a lot more money - I’d say there’s at least $100m in this place now. We have a huge annual budget because everybody here has been so successful in raising a lot of money from the government. We have several hundred people working in here, and I think it’s really changed the way chemistry and biology are done. Now we have all of these facilities and instruments and it’s changed the course of chemistry forever, in the sense that we no longer use test tubes and Bunsen burners, now it’s big lasers, big mass specs, big NMRs, big everything! So there’s no going back. It’s a little sad in some ways that we’ve lost the "individual investigator" approach, but I think we have to change to be competitive and really at the frontier of chemistry, biology and materials.’
In envisaging the Beckman Institute in the early 1980s, Gray was essentially predicting the situation that many UK and US chemistry departments are faced with today: expand or die. ’It’s really a question of how many research universities are going to be able to stay competitive, when the cost is so high’, he explains. ’Cambridge, Oxford, Imperial College, and so on in the UK probably have the resources to stay in there, but there are others which are going to fall by the wayside. Certainly in the US I don’t know how everybody can keep up. It’s so expensive to keep all of this stuff going at a high level, so I just don’t know what’s going to happen. Are we going to have just a few places left where you can get a PhD in chemistry? I know there are lots of discussions about this in the UK - about resources and how to sort them out. It’s so hard now.’
But if there is one thing that Harry Gray is good at it is seeing opportunity in every situation: ’There will probably be some reshuffling of people and departments may change. We may see things like departments of biophysics crop up as regular departments like chemistry and biology disappear. Maybe what’s happening at places like Queen Mary in London is what’s going to happen everywhere. Chemistry departments becoming ’science departments’ before breaking back out into more reasonable units. Maybe the system now is totally out of date and at Queen Mary they’ll get together and discover new connections. And that’s worth monitoring and seeing if that happens. The smaller places could lead the way. The big powerful places are so dug-in to their system that I don’t see them changing as quickly. The smaller places that have to change because of money and so on might just change and become something more interesting. So we should look to places like that for leadership.’
Prizes, prizes everywhere
Harry Gray has certainly had quite a career: from splashing acid on anything that cast a shadow, to demonstrating that electron tunnelling is the likely mechanism of respiration in life, setting up new ways of working and new institutes along the way, it is no wonder that he has recently been awarded the Wolf prize for chemistry. For someone so successful, though, he is genuinely modest. ’It’s certainly a very nice prize’ he comments, with understatement that verges on embarrassment. ’There are some very distinguished people who have won in the past. It came as a real surprise to me, to tell you the truth. You don’t expect to win these things really’. And I believe him.
Apparently when the organisers of the prize were trying to notify Gray that he had won, he was busy preparing a presentation for a conference. ’The phone in my motel kept flashing to tell me someone was calling and I didn’t pay any attention to it. Finally my wife got to me and said "you’d better answer the phone, I think you’ve won the Wolf prize!"’. This nonchalance shouldn’t be mistaken for ingratitude, though. Gray is genuinely thrilled. ’It is a nice prize - I’ve had a lot of connections with Israel: I was a professor there in 1979 and another prize I have, the Harvey prize, is from Israel. I’ve collaborated with people there for years so I have lots of friends there. I’m really looking forward to going back there and seeing everybody.’
Indeed Gray is very much in demand these days, having achieved near-celebrity status within the scientific community. Prior to this interview I mentioned to two US scientists that I was planning to meet Harry Gray. They laughed and replied ’in the States we just call him Harry’. And Gray really seems to enjoy this popularity. ’Oh yeah!, he laughs, ’I’m known everywhere as Harry. Like Barbra Streisand is know as Barbra. I’m just Harry.’
Delving into the unknown
Popular myth says that a Wolf prize is to a Nobel prize what the Golden Globes are to The Oscars. Gray is aware of this, and is trying to ignore it. ’A lot of people who have won the Wolf prize have gone on to win a Nobel prize. Quite a large number actually, but you can never tell about these things. I’m very happy to win the Wolf prize! What comes, comes. The big thrills, really, are doing the work. They come when you figure out something, or see something new that you didn’t expect. Winning prizes is just the icing on the cake. You have had all this great satisfaction from doing science and figuring things out and seeing things that nobody’s ever seen and that’s thrill enough! That’s why I think scientists are so very lucky, to be able to go into the unknown and see things for the first time.
’These prizes are really nice, but they’re not what you live for. But yes, in a way the pressure is on me: the last two people to win the Wolf prize from Caltech, Rudy Marcus and Ahmed Zewail, both went on to Nobel prizes. But I’m having so much fun working. And there are some things left which I really want to figure out.’
So what does the future hold for Harry Gray? At the moment he is working closely with Jay Winkler, trying to understand protein folding, using a lot of the methods he used to work out electron tunnelling. And he is particularly interested in the relationship between protein folding and disease. ’There are lot of terrible diseases which are due to proteins not folding properly. Top of the list is Alzheimer’s, next to that is Parkinson’s, type 2 diabetes, mad cow disease, it’s a long list! And so we have a tremendous number of powerful techniques, which we developed for studying electron tunnelling, and you can apply a lot of those to understanding protein folding. I’m very excited about going all out to see if we can discover the connection between protein folding and disease. I’d love to figure out Alzheimer’s.
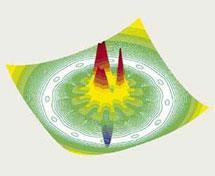
’We can make a start on it - we’ve done some really interesting work and written up the results of some of our first experiments in The Economist. We have very powerful methods using femtosecond and picosecond lasers to make movies of proteins as they are folding. We’ve also published the so-called landscape for protein folding, after we carried out some very powerful time-resolved experiments. But it’s the younger people here who are the leaders: Jay Winkler, Jennifer Lee and others in my group. But I can stay involved and contribute in certain ways. I’d like to make a connection with these terrible diseases, that might make a difference somewhere down the road. That would give me a great deal of satisfaction. It remains to be seen whether we can, but are having a lot of fun doing it!’






No comments yet