Five years after acrylamide's discovery in foods, industry is still hard at work trying to cut levels of the potential carcinogen in convenience products. Emma Davies investigates
Five years after acrylamide’s discovery in foods, industry is still hard at work trying to cut levels of the potential carcinogen in convenience products. Emma Davies investigates
Remember acrylamide? When Swedish researchers first identified the suspected carcinogen in foods in 2002, the mainstream media screamed food scare. Lists were soon compiled of foods high in acrylamide: the prime offenders turned out to be western favourites including crisps, potato chips, crackers, biscuits, bread, and coffee. Our favourite foods were going to kill us.

Could this simply be another food scare without substance? Despite extensive studies, there is still no evidence of a link between acrylamide in foods and cancer. That is not to say that acrylamide isn’t still a big research topic. Academics and industrialists are hot on the trail of the chemical, discovering exactly how it forms and coming up with new ways to cut its levels in foods.
Swedish shock
Margareta T?rnquist of Stockholm University led the team that first revealed the prospect of acrylamide in foods. She had compared blood samples from Swedish tunnel builders working with a sealant containing acrylamide with those taken from the general Swedish population. The results were astounding. Not only did the tunnel workers have high blood levels of acrylamide, but the other people tested also had enough acrylamide in their blood to suggest that they were regularly exposed to the chemical.
Suspicion soon turned to the food supply and Tornquist discovered acrylamide in carbohydrate-rich baked and fried foods.
Researchers’ worst fears were confirmed when rat feeding studies revealed that acrylamide increased the rates of several types of cancer including testicular mesotheliomas and mammary gland and uterus adenocarcinomas. To make matters worse, acrylamide is a known neurotoxin. ’If you were working in a lab making polyacrylamide gels, you would take an awful lot of care,’ says Bronek Wedzicha, head of food science at the University of Leeds.
Lorelei Mucci from the Harvard School of Public Health in Boston, US, has used population-based studies to search for a possible link between dietary acrylamide and the risk of several cancers including colon, rectal, kidney, bladder and breast. So far she has found no association between a high intake of foods containing acrylamide and cancer. It remains to be seen whether dietary acrylamide has neurotoxic effects.
Mucci acknowledges that the ’apparent lack of association between dietary acrylamide and cancer risk in humans appears to contradict the findings from the animal feeding studies’. She has several theories to explain this. First, the rats were exposed to acrylamide levels 1000 to 100,000 times higher than human exposure - the mean dietary intake of acrylamide in adults averages 0.5 micrograms per kg body weight per day. Humans may also effectively detoxify acrylamide at dietary levels, she suggests. What is more, the rats were given acrylamide in solution, not in foods. ’It is unclear the extent to which acrylamide is bioavailable in foods,’ she adds.
Mucci’s data are in line with other work, including a key epidemiological study by Claudio Pelucchi and his team at the Instituto di Richerche Farmacologiche Mario Negri, Milan, Italy, which found no positive association between cancer risk and dietary acrylamide.
Wedzicha is among those who doubt that foods containing acrylamide pose a rise to health: ’I haven’t seen the evidence to say that in foods it actually harms us. There may be a long-term issue with what happens to us in old age.’ Many researchers, however, remain concerned by the possible risks posed by acrylamide. ’If we care about toxic substances like nitrosamines in foods, we must care a lot about acrylamide because you find it in almost every heated product,’ says Thomas Amrein, from ETH Z?rich, Switzerland.
Scientific attraction
Most researchers in the field agree that acrylamide has been fascinating to study, from unearthing its formation mechanisms to studying the reaction kinetics.
’The interesting thing is that there is only a remote chance that anyone using existing technologies five years ago would have discovered acrylamide accidentally,’ says Wedzicha. ’You’ve got to go through a derivatisation process in order to spot it using gas chromatography-mass spectrometry.’
We now know a great deal about how acrylamide forms in foods; it is universally acknowledged that the amino acid asparagine is the main source (see box). The finer mechanistic details of acrylamide formation remain the subjects of much lively debate between researchers in the field. The discussions are largely academic and Wedzicha, who was in the UK team that first pinpointed asparagine as the acrylamide source, questions whether researchers should get ’hung up’ with the mechanism itself or whether the reaction kinetics are more important. ’The identity of the intermediates can be important but it’s not crucial,’ he says. ’We can gloss over some of the refinements.’
’Acrylamide is a brilliant case study,’ says Wedzicha. He suggests that knowledge gleaned from acrylamide research should be used to predict which other thermally generated toxicants foods contain. ’How one addresses the formation of toxicants in the future is really quite a challenging intellectual problem,’ he says. Amrein agrees: ’You learn from acrylamide as a system what you can try and you can develop a kind of systematic approach which you can copy and paste to new problems.’
Industry initiative
Understanding the acrylamide basics has allowed industry to make real steps to cut levels of the chemical. ’The food industry has committed to reducing acrylamide levels where it can,’ says Richard Stadler, quality manager at Nestl? Product Technology Centre in Orbe, Switzerland. ’There has been success in cutting acrylamide levels, for example in crispbreads, French fries, potato crisps and in certain bakery products,’ he adds.

Steps taken so far have not required major alterations to production lines. Changes include tweaking cooking times, lowering pH and adding new ingredients.
In some cases, ammonium bicarbonate, commonly used as a raising agent in baked goods such as gingerbread, is replaced with sodium bicarbonate. Ammonium bicarbonate leads to faster breakdown of certain sugars, promoting the formation of reactive carbonyl compounds and giving higher acrylamide levels. Industry is, however, conscious of the fact that adding sodium bicarbonate raises general sodium levels.
Adding alternative amino acids to compete with asparagine is a popular research option, with much attention focused on glycine. ’The jury is out on glycine,’ says Peter Ashby, Cereal Partners Worldwide. ’Glycine can form formaldehyde when heated [through Strecker degradation] and to use it at the factory level would be difficult.’
Meanwhile, a team led by Hans Blom from the Norwegian Food Research Institute in Oslo, has come up with a novel way to reduce acrylamide formation using lactic acid bacteria to metabolise reducing sugars to lactic acid. The team has managed to reduce significantly acrylamide levels in French fries, although the fries have a paler and less appealing appearance than normal.
How does acrylamide form?
In 2002, Nature published two papers proposing mechanisms for acrylamide formation (see Further reading). One was authored by UK food scientists Bronek Wedzicha from Leeds University and Donald Mottram and Andrew Dodson from Reading University.
Wedzicha had visited Reading shortly after acrylamide was first revealed in foods. At that time people were suggesting that the chemical must come from fat, linking its three carbons to those of glycerol and glycerides.
Mottram recalls: ’Bronek and I went out for dinner and we both said: ’’This isn’t right; what do we know about the Maillard reaction?’’ Products of the Maillard reaction between amino acids and reducing sugars such as glucose give much of the flavour and colour generated during baking and roasting.
Mottram and Wedzicha started to think in terms of the Strecker degradation, a reaction associated with Maillard that gives aldehydes through decarboxylation and deamination of amino acids. ’We did some workings on the restaurant’s paper table cloth and said: ’’That’s it! The acrylamide must come from asparagine’’. We designed experiments and confirmed our hypothesis,’ says Mottram.
The other Nature paper was by a team from the Nestl? Research Centre in Lausanne, Switzerland, led by Richard Stadler. It also pointed to a mechanism involving asparagine in the presence of reducing sugars or a suitable carbonyl source as the key to acrylamide formation.
It is perhaps not surprising that asparagine is the culprit. The amino acid is a vital component of many plants, where it is used to transport and store nitrogen.
There is still some debate as to the exact mechanisms of acrylamide formation. Mottram and Wedzicha believe that alpha dicarbonyls are necessary coreactants for acrylamide to form (see figure). Meanwhile, Stadler and his team back a theory that glycoconjugates such as N-glycoside formed in the early phase of the Maillard reaction are key intermediates. Both groups have shown the importance of asparagine’s Schiff base, which is decarboxylated to form intermediates that can go on to release acrylamide.
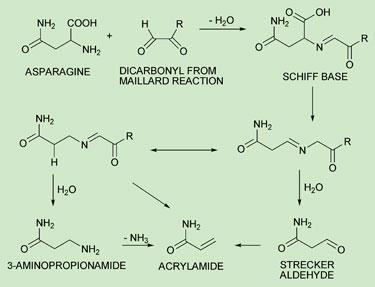
Many researchers are excited by the findings of Peter Schieberle, chair for food chemistry at the Technical University of Munich, Germany, who suggests that the biogenic amine 3-aminopropionamide (3-APA), also forms from asparagine and is a ’potent precursor’ of acrylamide.
Schieberle suggests that 3-APA is much more effective than asparagine at generating acrylamide. What is more, no carbohydrate or reducing sugar is needed because acrylamide forms from a simple thermal elimination of ammonia from 3-APA. ’Because of 3-APA’s high efficacy in acrylamide formation, the amine might serve as an indicator for the potential of a given food to form acrylamide, particularly for foods which are reheated by consumers,’ he says.
Enzyme hopes
Many researchers are excited at the prospect of using asparaginase, an enzyme which limits acrylamide production by breaking down asparagine. ’Industry has done a lot of work on asparaginase. We are doing preliminary work at laboratory scale
to see if it is effective - it could be a pretty hot tool to use,’ says Stadler.
Asparaginase doesn’t require any recipe changes and, according to Amrein, doesn’t affect the flavour of foods. ’It is the only approach that solves and does not fight the acrylamide problem,’ he says. The enzyme does have drawbacks - it can only be used in certain products, generally those that start off as a mix, batter or dough, and when it comes to breads, it is not suited to wholegrain products. What is more, excessive heat will inactivate the enzyme.
Asparaginase is still awaiting regulatory clearance for food use. Several companies have already filed for approval and some countries are expected to grant it this year. Several companies are developing asparaginase derived from genetically modified organisms (GMOs), which would be far cheaper, says Amrein.
Beyond this, the food industry has almost run out of further acrylamide reduction moves to make. ’We will continue looking for options, but in some products it will not be possible or very difficult to find a solution,’ says Stadler. A technology jump is needed to reduce significantly acrylamide levels, he says. This view is echoed by Bob Foot, from the European Snacks Association. ’We are getting to the point where technology won’t go any further and we are looking to the agro people to answer questions such as: ’’How can we get new potato varieties?’’’
Chemists are already working with agronomical teams. For example, a collaboration between Don Mottram’s team at the University of Reading, UK, and researchers from Rothamsted Research recently revealed that flour from wheat grown in sulfate-depleted soil contains far more asparagine (Chemistry World, December 2006, p29) and therefore gives higher acrylamide levels. Sulfate levels in soils have dropped dramatically in recent years because of changes in farming methods and a drop in atmospheric sulfur levels.
Savour the flavour
One of the factors holding industry back is the link between acrylamide formation and flavour generation. Reducing one without affecting the other is a tricky, almost impossible task. Flavour volatiles such as pyrazines and aldehydes are formed by the same Strecker reactions that give acrylamide, says Wedzicha. ’At least, that’s the na?ve view of it. Imagine you’ve got a Maillard reaction pathway that gives colours and flavours and just coming off that is the acrylamide bit.’
Mottram’s team has proposed using glycine and citric acid together to cut acrylamide while minimising the effects on volatile flavour compounds. Citric acid limits the generation of certain flavour compounds such as alkylpyrazines, whereas glycine promotes their formation. So using the compounds together almost cancels out their negative aspects.
Mottram is currently studying the relationships between the pathways of acrylamide and flavour formation to see if he can decouple them from each other.
Share and share alike
Acrylamide researchers in industry and academia are very open with each other and freely share data. In many countries, government bodies are also helping to fund and disseminate research. As Amrein points out: ’No-one is to blame for acrylamide in food - it’s naturally occurring and therefore everyone should stay on the same side’.
One way that industry is sharing information is through the CIAA (EU Confederation of food and drink industries) toolbox, a document which suggests options for industry to take to cut acrylamide levels. The toolbox was set up in 2005 and is continually updated. ’We’re very proud of it,’ says Stadler. It is marketed particularly at small companies that do not have the manpower or the budget to take acrylamide levels into consideration.
Meanwhile, an academic team led by Wedzicha is developing its own industry toolkit, this time based on reaction kinetics. ’We’ve done something really remarkable here,’ enthuses Wedzicha. Working with Mottram’s research group, the team has created a kinetic model that allows it to predict, from the way that acrylamide forms in, say, potatoes, how it forms in wheat or rye. The team is currently having a shot at using the same parameters to predict the amount of acrylamide in coffee. ’Under some circumstances it works: it’s amazing,’ says Wedzicha.
Together with Andy Taylor’s group at Nottingham University, UK, which uses gas-phase analysis to analyse acrylamide and related compounds in real time, the researchers have a grant from the UK Food Standards Agency to produce the toolkit, which should be ready for industrial use by the end of the year.
Weighing up the problem
Most experts in the field talk about the need to put the potential risks posed by acrylamide into perspective. Acrylamide-rich foods are often high in fat and calories too, raising the issue of obesity. ’I think that the problem associated with obesity from eating fatty foods is a much greater problem than acrylamide,’ says Mottram.
When epidemiologist Mucci’s first study came out, finding no link between dietary acrylamide and cancer, it was widely called the French fries study. ’People came out saying: ’’French fries are good for you,’’’ recalls Mucci. ’It was really hard to get the public health message out that acrylamide in the diet in the amount that’s consumed doesn’t appear to be related to cancer. However, fried foods are still associated with a higher risk of obesity and we know that this is an incredibly important public health problem. We know that obesity is associated with an increased risk of cancer. Reducing obesity would be an important way to reduce cancer in the population.’
So why exactly is industry ploughing so much money and effort into acrylamide research? Ask anyone in industry and they seem shocked at the question. They are almost unquestioningly following recommendations from bodies like the World Health Organisation.
There is still the possibility that acrylamide could be shown to be harmful. After all, we are all exposed to the chemical at some level. Removing it from our diets would be nigh on impossible, unless we resign ourselves to eating bland and uncooked foods. Although perhaps a bland food future could go some
way to helping solve the global obesity crisis.
Further Reading
D Mottram et al and R Stadleret al, Nature, 2002, 419, 448 and 449
R Stadler et al J. Agric. Food Chem., 2004, 52, 5550
M Granvogl and P Schieberle, J. Agric. Food Chem., 2006, 54, 5933
Acrylamide action
Until food authorities are satisfied that there is no link between dietary acrylamide and cancer, they will continue to monitor and curb acrylamide levels.
The Food and Agricultural Organisation (FAO) and the World Health Organisation (WHO) have been involved in the risk assessment of acrylamide from the start. In 2005, the joint FAO/WHO expert committee on food additives and contaminants reported that acrylamide may be a ’human health concern’. The experts recommended that efforts to reduce acrylamide levels in foods should continue. ’We take this recommendation very seriously,’ says Richard Stadler, quality manager at Nestl? Product Technology Centre in Orbe, Switzerland.
In Europe, Germany currently takes the toughest stance on acrylamide. Its food safety authority has introduced a ’minimisation principle’, which recommends that companies producing foods with acrylamide levels in the top 10 per cent in a food category reduce levels as much as possible. Using monitoring data, it contacts industries producing products with high acrylamide concentrations. The authority periodically recalculates and reduces recommended acrylamide levels. The system has its drawbacks, as Stadler points out. ’At a certain point in time, you’re going to reach a platform where you have picked all the low hanging fruits and you can’t go any further,’ he says.
The European Commission has been involved in monitoring acrylamide levels through various groups. In 2002, the Joint Research Centre (JRC) began to set up quality control measures to check the reliability of data on acrylamide in foods.
The Institute for reference materials and measurements (IRMM) carried out ’proficiency tests’ on a variety of different food types, says Thomas Wenzl, an operating manager at the IRMM. The JRC also set up a database for acrylamide information and research which is published on the IRMM website. ’The database was designed in close collaboration with representatives from the food industry and more than 9,000 data have been received,’ says Wenzl.
The IRMM also reviewed and analysed the analytical methods available for determining acrylamide levels.
Much EU work goes through the Heatox project, set up to identify, characterise and minimise heat-generated food toxicants.
In the UK, the Food Standards Agency (FSA) funds a range of acrylamide research projects. The main aim of these activities is to minimise the amount of acrylamide present in food, says the FSA.
The US Food and Drug Administration continually measures acrylamide levels in foods under its total diet study. It also initiated carcinogenicity and neurotoxicity studies, which will be completed this year.






No comments yet