Billions of people owe their lives to our ability to grab nitrogen out of the air to fertilise our crops. But there can be too much of a good thing, reports Kira Weissman
Billions of people owe their lives to our ability to grab nitrogen out of the air to fertilise our crops. But there can be too much of a good thing, reports Kira Weissman
Whether you’re a plant or an animal, a square meal includes a good dose of nitrogen. Yet most nitrogen on Earth is locked up in atmospheric dinitrogen (N2), none of which can be used by higher organisms to help them grow. The reason for this is the strong triple bond that holds the two atoms together, rendering N2 stubbornly unreactive. Nitrogen can only sustain life when it is converted into reactive forms such as ammonia (NH3) or nitrate (NO3-), making it a limiting nutrient for plant growth in many native ecosystems. So to boost crop yields, farmers have long fertilised their land with various forms of pre-activated nitrogen, including bird droppings, animal manure and saltpetre (potassium nitrate).

By the end of the 19th century, feeding 1.6 billion people had largely depleted these natural fertilisers. With mass starvation looming, Sir William Crookes, the president of the British Association for the Advancement of Science, exhorted chemists to solve this nitrogen predicament. And two German scientists, Fritz Haber and Carl Bosch, answered Crookes’ call. In 1908, Haber invented a way to artificially ’fix’ atmospheric dinitrogen, turning it into ammonia using the combined effects of high temperature (about 500?C), pressure (about 200atm), and an iron oxide catalyst to coax the N2 into reacting with hydrogen gas (H2). It was left to Bosch to scale-up the synthesis for industrial production.
The invention was nothing short of world changing, earning its developers the Nobel prize (Haber in 1918 and Bosch in 1931). But like the Roman God Janus, the process has two opposing faces. Most Haber-Bosch ammonia ends up in fertiliser, providing food for almost half of humanity.1 In fact, thanks to Haber and Bosch, our ability to feed the world will never again be limited by nitrogen. Yet as the raw material for explosives, reactive nitrogen has fuelled conflicts around the globe. And evidence is mounting for another grim consequence of all this extra nitrogen: the unbalancing of the global nitrogen cycle, which threatens both the environment and human health.2
Breaking the cycle
For much of human history, the nitrogen cycle has kept a lid on the amount of reactive nitrogen in the biosphere (see box). But that has changed over the last few decades, as we’ve ramped up our use of atmospheric N2. Human activity is now estimated to create 190 teragrams (one teragram = one million tonnes) of reactive nitrogen every year. To put this into context, all natural terrestrial processes combined fix about 100 teragrams. ’So on a global basis, humans are creating about twice as much reactive nitrogen as the natural system,’ explains James Galloway, professor of environmental sciences at the University of Virginia, US.
In a spin
’If you take humans out of the equation, the nitrogen cycle is pretty straightforward,’ explains James Galloway, professor of environmental sciences at the University of Virginia, US.
Nitrogen naturally enters the biosphere in one of two distinct ways. The more dramatic, though far less significant, is via lightning - each bolt breaks apart a small amount of N2 to form nitric oxide (NO); the NO is oxidised to nitric acid (HNO3), which washes from the sky as acid rain. However, most nitrogen fixation is carried out by a small group of soil bacteria called diazotrophs, in a biological version of the Haber-Bosch process. Of the millions of species on the planet, only about one thousand posses this ability.
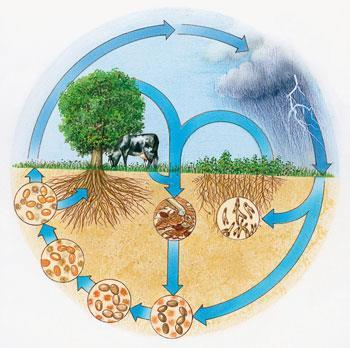
Some of the most important microbial fixers of nitrogen in the soil are members of the genus Rhizobium, which live in intimate symbiosis with plants. Tucked up in root nodules of leguminous plants (such as broad beans, garden peas, alfalfa and peanuts), they deliver NH3 directly to the plant, in exchange for nutrients. Nitrogen then continues its journey through the biosphere when fungi and animals eat the plants or other animals, taking up nitrogen from these foodstuffs in the form of amino acids, nucleotides and other small molecules.
Once assimilated into plants and animals, much of it within proteins and amino acids, a large portion of this reactive nitrogen stays within the bioavailable pool, because when plants and animals die, decomposers such as bacteria and fungi efficiently release the nitrogen back into the soil as ammonia. Much of the ammonia is then converted into nitrate (NO3-) by two groups of nitrifying bacteria, making fixed nitrogen available again to plants, including non-legumes. However, nitrogen does eventually exit the living world through yet another microbial process called denitrification, which closes the nitrogen cycle: bacteria living in the deep soil convert reactive forms of nitrogen back into gaseous N2, which then escapes into the atmosphere.
A similar cast of characters - nitrogen fixers, consumers and degraders, nitrifiers and denitrifiers - makes life possible in the open oceans. This parallel web of nitrogen exchanging reactions is largely isolated from its terrestrial counterpart, although a small extent of mixing does occur where rivers dump into coastal waters, bringing a cargo of leached soil nitrates with them.
Only 125 teragrams of this extra nitrogen originate with the Haber-Bosch process, however. For centuries people have known that if beans and peas are planted in a field, the next crop does better. Galloway has estimated that this strategy of using legumes to ’pump’ nitrogen into the soil now accounts for an additional 40 teragrams of fixed nitrogen annually. A further 25 teragrams come from fossil fuels. Burning these ancient materials at high temperature and pressure converts organic nitrogen in the fuel, as well as N2 in the combustion chamber, into nitrogen oxides (NOx). These molecules can travel thousands of miles before falling back to earth as acid rain.

The natural nutrient cycle is most unbalanced in regions where agriculture, industry and fossil fuel consumption overlap, such as Europe and North America. But with fertiliser and energy use on the rise in the developing world, particularly in China and India, nitrogen overload is quickly becoming a global issue, says William Bowman, professor of ecology and evolutionary biology at the University of Colorado, US. The problem is that while ecosystems can absorb some additional nitrogen by increasing plant biomass and rates of denitrification, they eventually reach saturation, bleeding off the nitrogen surfeit into the surrounding soil, air and water. Each molecule of excess nitrogen lost to the environment can have a series of linked, negative impacts - a phenomenon which Galloway and colleagues have dubbed ’the nitrogen cascade.’
As NOx in the lower atmosphere (troposphere), nitrogen promotes formation of photochemical smog, a noxious mixture of ozone (O3) and particulate matter. Low-lying O3 induces a number of respiratory ailments, including reactive airway disease and asthma, while fine airborne particles are linked to cardiovascular illnesses and increased mortality.5 Swept onto the soil in acid rain, nitrogen acidifies semi-natural, nitrogen-limited ecosystems such as forests and grasslands. Acidification occurs ’directly through the generation of excess protons’, explains Bowman, ’but also indirectly by enhancing the loss of soil cations that act as buffers.’ Toxic ions such as those of aluminium and iron gradually replace vital plant nutrients such as calcium, magnesium, potassium and sodium6 - leading to fertility drops and weed species invade. This species shift can propagate through the food-chain, changing the structure of the entire ecosystem. The resulting negative effects on biodiversity are evident in the Netherlands, reports Jan Willem Erisman of the Energy Research Centre of the Netherlands, where nitrogen deposition rates have been among the highest in the world. Grasslands which used to boast sixty species now have only two, he says.

The surplus nitrogen can also make its way into the waterways, as soil acidity enhances leaching of nitrate into surface and ground waters.7 Drinking this contaminated water, says Bowman, has been linked to methemoglobinemia or ’blue baby syndrome’, a condition in which oxygen is not effectively transported in the blood. And when the nitrogen carries on its journey into wetlands, ponds and lakes, nutrient over-enrichment (’eutrophication’) provokes a frenzy of plant growth, and algal blooms. When the algae die off, they are eaten by microbes, which consume vital oxygen in the process. If nitrogen inputs continue, all creatures which cannot swim away will eventually suffocate.
Conveyed to the coast by rivers, reactive nitrogen ultimately targets estuarine regions, such as bays and sounds, where oxygen depletion can cause ’dead zones’ - areas of bottom waters too oxygen-depleted to support most ocean life - which may extend for thousands of square miles around river outlets. A single dead zone can claim thousands of tonnes of marine life, robbing commercial fisheries of millions of dollars. At last count researchers had spotted more than 400 of these regions in the world,8 and the number is growing, reports Bowman.
Even the open ocean is not immune to anthropogenic nitrogen’s effects. In fact, Robert Duce of the departments of oceanography and atmospheric sciences at Texas A&M University and colleagues, have estimated that nitrogen deposited from the air is currently responsible for approximately 3 per cent of new marine life in these regions.3 This sounds like a good thing because in order to build these organisms, the ocean absorbs 10 per cent of man-made CO2, a notorious greenhouse gas. However, two-thirds of this reduction may be offset by the greater release of nitrogen back into the atmosphere in the form of nitrous oxide (N2O), which is 300 times more powerful as a greenhouse gas than CO2.
This ruinous cascade can continue for years and even centuries, until the reactive nitrogen reaches an environment where denitrification can occur, and it rejoins the atmosphere as dinitrogen, says Galloway. And the impacts aren’t limited to the nitrogen cycle, as reactive nitrogen interacts with the planet’s carbon cycle, now recognised to be the major driver of global climate change over the past century.9
Our nitrogen future
As Earth’s population is expected to reach 9 to 10 billion in the coming decades, our creation of fixed nitrogen only looks set to accelerate. Can we manage to produce enough food and energy to support our growing population, without further damaging the environment and our health?

Environmental scientists argue for a three-pronged approach to tackling the problem: reducing the creation of reactive nitrogen, increasing the efficiency with which it’s used, and converting it back to atmospheric dinitrogen.4 The first target, Galloway insists, should be fossil fuels: ’If you don’t form the reactive nitrogen during combustion, it can’t contribute to the nitrogen cascade. That’s just a no-brainer.’ The know-how for minimising NOx emissions is already in place, he says, so it’s simply a matter of enforcement at the public policy level. Savings here could amount to 18 teragrams per year.4
As there is no substitute for fixed nitrogen in agriculture, however, the aim is to enhance efficiency. Galloway has calculated that although we put 140 teragrams of nitrogen into food production, Earth’s population could make do with 13, if only the reactive nitrogen we created could be used with 100 per cent efficiency. In other words, each year we create approximately an order of magnitude more nitrogen than we need. The root of the problem is that getting nitrogen into plants and animals is an inefficient process: ’When a person sits down to eat a pork chop,’ Galloway explains, ’only about 20 per cent of the nitrogen used in making that food actually enters the person, while the other 80 per cent is lost during production - making the feed, making the animal, slaughtering the animal, and making the pork chop.’ Adding to this, says Erisman, is the deliberate over-application of fertilisers: ’Farmers are accustomed to very cheap fertiliser, so they use it as a form of insurance. If you just put on enough fertiliser, you ensure optimal yields.’
Thus, huge gains in nitrogen efficiency can be made through simple nitrogen management strategies: tailoring fertiliser composition and quantity to a particular crop and timing its application, optimising animal feed, and recycling the nitrogen that livestock excrete back into the system by using manure as fertiliser. The Netherlands road-tested these recommendations with its 1998 Mineral Accounting System, which provided farmers with customised fertilisation schemes to reduce nitrogen losses and optimise use. The result, according to Erisman, was a 40 per cent reduction in fertiliser consumption, without a major drop in crop yields. If implemented worldwide, such approaches could reduce demand for reactive nitrogen by 30 teragrams each year.4

One of the main barriers to putting these fixes into action on a larger scale is that many people simply aren’t aware of our reactive nitrogen troubles. Fortunately, a growing number of scientific organisations are banding together to alert governments to the threat. For example, the European Science Foundation’s Nitrogen in Europe project aims by 2011 to furnish policy makers with a document called the European Nitrogen Assessment, containing a comprehensive account of Europe’s nitrogen problems and proposing long-term solutions. Stateside, the US Environmental Protection Agency has stepped up efforts to control nitrogen pollution, primarily for the more industrial east, reports Bowman. And there are already encouraging signs that politicians are listening: the Convention on Long Range, Trans-boundary Air Pollution, whose 51 members include Europe, the US and Canada, recently established a task force to deal with the reactive nitrogen issue.
Scientists are also targeting the end consumer. For example, Galloway, Erisman and colleagues are developing a web-based ’nitrogen footprint model’ called N-Print, the nitrogen equivalent of the carbon footprint. Based on people’s energy and food consumption patterns and where they live, the program will tell them not only how much nitrogen they are using, but how much of it is lost to the environment. The hope is that by arming the public with this information, individuals will start to minimise their impacts. According to Galloway, even small changes can make a difference: reducing energy consumption at home, travelling less often, and eating less meat (particularly beef) and more vegetables.
Action is urgently needed. A century on from Haber’s discovery, scientists increasingly recognise that the very same reactive nitrogen which ended humanity’s food crisis is provoking a large-scale environmental catastrophe. Only time will tell whether we can match Haber’s ingenuity with new, world-changing inventions that will allow us to grow and sustain human life without sacrificing
our environment.
References
1 J W Erismanet al, Nat. Geosci., 2008, 1, 636 (DOI: 10.1038/ngeo325)
2 An animation providing an overview of the sources and effects of reactive nitrogen compounds is available on the website.
3 R A Duce et al, Science , 2008, 320, 893 (DOI: 10.1126/science.1150369)
4 J N Galloway et al, Science, 2008, 320, 889 (DOI: 10.1126/science.1136674)
5 A R Townsend et al, Front. Ecol. Environ., 2003, 1, 240
6 W D Bowman et al, Nat. Geosci., 2008, 1, 767 (DOI: 10.1038/ngeo339)
7 Industrial Ecology and Global Change (R Socolow, C Andrews, F Berkhout and V Thomas, Eds), 1977, Cambridge University Press
8 R J Diaz and R Rosenberg, Science, 2008, 321, 926 (DOI: 10.1126/science.1156401)
9 N Gruber and J N Galloway, Nature, 2008, 451, 293 (DOI: 10.1038/nature06592)






No comments yet