A failed antidepressant has been shown to increase women's sex drive
A new drug being developed by Boehringer Ingelheim could give a boost to the sex drive of women with low libido. The drug, known as flibanserin, has been shown in clinical trials to increase their sexual desire when taken once a day at bedtime.
The results from four pivotal Phase III clinical trials on women with hypoactive sexual desire disorder (HSDD) were presented this week at the European Society for Sexual Medicine’s congress in Lyon, France. The trials showed that participants taking flibanserin had a significant improvement in their sexual desire compared to those given a placebo. They also experienced less of the distress associated with sexual dysfunction.
The drug was initially being investigated as a treatment for depression, and acts on the serotonin receptors in the brain - it is both a 5-HT1A receptor agonist and a 5-HT2A receptor antagonist. It is also a partial agonist at the dopamine D4 receptor.
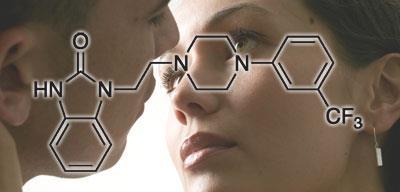
Neurotransmitters such as serotonin are believed to be involved in sexual function, and antidepressants are commonly associated with a loss of libido, so this was an obvious side-effect to look out for during clinical trials in depression. But far from suppressing the libido in women, it appeared to have the opposite effect, so trials in women with HSDD were initiated.
Hormone replacement can improve the libido of women who have had their ovaries removed, but there is no available drug to treat those who have not. There have been accusations that pharma companies invent new diseases like HSDD in order to sell more medicines, but according to Kathleen Segraves, an assistant professor at Case Western Reserve University in the US who has worked in the field of sexual functioning for many years, this is not the case here. HSDD is a very real disorder, she says, and the potential for a treatment for these women is very exciting.
’What is so interesting [about the condition] is that women who present with clear HSDD are often in good, loving and caring relationships,’ she says. ’The low desire is not related to depression, and except for the relationship bond with their partner the woman would be OK if she never has sex. Many describe the loss of desire as ’I want to want to have sex’, so the study findings are potentially extremely important.’
She adds that tabloid accusations that the drug will turn women into insatiable sex divas are wide of the mark. ’Evaluating the findings must be seen in the perspective of the population of women with low libido,’ she says. ’If a woman has had zero thoughts about engaging in any sexual activity for months or years, that is a big personal change for that woman. The women I see in therapy often see themselves as broken. HSDD is a very prevalent sexual problem, and women who seek therapy are very distressed by the lack of sexual desire. This drug may help many women with low libido, but may also be the first of many drugs working on the CNS to modify libido.’
However, even if HSDD is a genuine medical condition, there is still debate about whether it is appropriate to treat it with drugs. ’It’s clear that men, in general, desire to have sex more often than their partners, and this may make women feel that they?ve got a problem,’ says David Webb, Christison Professor of therapeutics and clinical pharmacology at the University of Edinburgh. ’So would they be taking this pill for their partners rather than themselves? Would it not be better to address the psychological problem or sort out the relationship rather than sticky tape over it with a pill?’
He adds that, as a pharmacologist, he is also concerned about the large placebo effect that was evident in the trials. ’You would want trials to be done to show it was a true benefit, and not just one associated with an antidepressant,’ he says. ’And because it has effects at more than one 5-HT receptor, there may be safety issues and issues with dependency and withdrawal. A side effect that occurred in one in 10,000 patients would not show up in clinical trials, but might prove devastating in the first few months after the drug is launched.’
Sarah Houlton






No comments yet