Commercially available magnets could significantly improve the efficiency of water splitting in a microgravity environment. These types of device would be used to supply astronauts with oxygen during long-term missions but they are less effective in low gravity. The international team of researchers, from the US, UK and Germany, who made the discovery, also developed two proof-of-concept devices which they claim could, with further testing in low gravity environments, be used in future space missions.

Ensuring astronauts have access to a reliable and continuous supply of oxygen during deep space missions has been a challenge since the early days of space exploration in the 1960s. Currently, in microgravity environments oxygen is produced using electrochemical water-splitting, which requires complex mechanical components and a significant amount of energy. The process is further complicated by the fact that in the weightlessness of microgravity, gas bubbles do not float upwards like they do on Earth. Instead, they tend to stick to the surface of the electrode, inhibiting the reaction.
Putting Mars within reach
To address these challenges, the team set out to engineer electrochemical cells that would help to simplify the way oxygen is generated in space. ‘In the case of a Mars transit mission, the reliability of the oxygen production system … is still not high enough to support a long-term mission,’ explains Álvaro Romero-Calvo, an aerospace engineer at the Georgia Institute of Technology and a member of the team. ‘The problem is that there are so many moving components and centrifuges and pumps and hydrogen sensors and so on in a closed loop operating in reduced gravity, that all those spare components stack up and add a lot of mass to your system.’
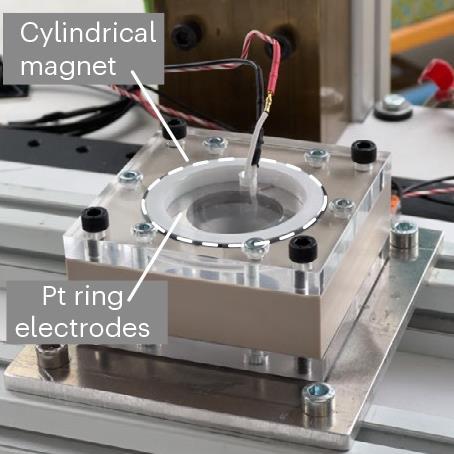
Incorporating off-the-shelf neodymium magnets into electrolysis devices, the team developed a passive phase separation system that pushed the bubbles away from the electrodes and collected them at designated spots. Romero-Calvo explains that there are two main forces being demonstrated in their work: diamagnetic force and magnetohydrodynamics. The magnetohydrodynamic effect works to improve gas bubble detachment from the electrode surface causing them to swirl around, while the diamagnetic effect helps to direct the gas bubbles to specific collection points.
‘What we’re ultimately trying to do is to separate gas bubbles – oxygen and hydrogen – from the water or the electrolyte, without moving parts or centrifuges or pumps or anything like that,’ says Romero-Calvo.
To investigate the impact of these two forces the researchers used a drop tower to generate brief periods of microgravity during free fall lasting a total of 9.3 seconds. Using this approach, they found that the magnet enhanced water electrolysis with current density improvements of up to 240% in microgravity, compared with electrolysis devices without a magnet.

To exploit these effects, the researchers developed two simple proof-of-concept devices – one a proton-exchange membrane electrolyser cell that uses diamagnetic forces for efficient oxygen and hydrogen gas collection and the other a magnetohydrodynamic drive cell that causes vortical gas–liquid phase separation. In microgravity, they found that the devices achieved water splitting with an efficiency close to that achievable on Earth.
‘The goal of these two proof-of-concept devices is, can we induce gas separation in microgravity to produce oxygen and separate it within the same device … in a very simple way?’ says Romero-Calvo.
Long-term testing
The team are now looking to assess the long-term performance of the system. ‘We need long-term microgravity conditions, either through a suborbital rocket, which is something we’re going to launch in the near future, or through orbital experiments,’ he adds. ‘The second part is that many innovations that happen at the electrode or the cell level … work very well in a tiny, little cell but when you try to make it fit for astronauts for six months, it doesn’t really work that well. So, the scale up is something we’re working on as well.’
Mark Symes, an electrochemist at the University of Glasgow, described the work as ‘super cool’ and ‘a tour de force’ of doing electrolysis in difficult conditions. ‘Electrolysis in space is a huge issue, particularly if you’re going to go and live or have a semi-permanent base on the moon or Mars, you’re not going to want to ferry oxygen backwards and forwards from Earth endlessly,’ he explains.
‘What’s really cool about this magnetic work, is there are no moving parts, so you just have a standard electrolyser with a magnet. Basically, the magnetic field is producing a convection and a sort of jitter that pushes these bubbles off before they get too large.’
However, Symes says he was left with several questions about the work. ‘I didn’t see, at least in the main paper, that they measure the purity of those gases, and that’s obviously something that you would want to do; you would want to make sure that your level of hydrogen in your oxygen was below the explosion limit, otherwise you wouldn’t want to put that on the spacecraft.’
‘The other question I had was around their current density, so the rate at which they can make gas per unit area of electrode,’ he adds. ‘For both the devices … they’re very much towards the lower end of what you’d want – 150 milliamps per centimetre squared. It’s not great – for reference, a conventional electrolyser on Earth would run at least an amp per centimetre squared, so at least 10 to 20 times more. So, you’d want to boost that current density whilst keeping the gases separate.’
References
Ö Akay et al, Nat. Chem., 2025. DOI: 10.1038/s41557-025-01890-0















No comments yet