Discovery of the normal healthy function of proteins that malfunction in Alzheimer's disease points to possible treatments.
A? is a short peptide that is cut out of the larger protein APP (?-amyloid precursor protein), which then forms aggregates known as amyloid fibrils that characterize Alzheimer’s disease pathology. Similar aggregates formed by other peptides or proteins are associated with a range of other diseases. Researchers in Germany are now closer to understanding the natural function of one of the two enzymes that cut A? out of APP, and have recreated the process by which fibril formation begins in vivo.
APP plays a vital role in controlling the concentration of metal ions in cells, but the A? fragment only occurs in a disease context. Thus, a key approach to finding a cure for Alzheimer’s disease is targeting the two enzymes that make the cuts resulting in A? production, known as ?-secretase and gamma-secretase. Christian Haas and colleagues at the Ludwig-Maximilians-University in Munich, and Alistair Garratt’s team at the Max-Delbr?ck-Center for Molecular Medicine in Berlin, in collaboration with other US and European researchers, have now discovered that the ?-secretase enzyme, also known as BACE1, plays a crucial role in new-born mice.1It takes part in the control of myelination, whereby nerve cells are coated in a protective myelin layer.
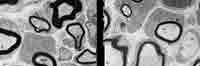
While the precise details of the BACE1 function remain to be explored, Garrett explains that his research already presents ’evidence that this is mediated through proteolysis of Neuregulin-1, a molecule which controls myelination in the peripheral nervous system.’
Haass and Garratt agree that despite the requirement of BACE1 function for myelination, this enzyme remains an attractive target for treating Alzheimer’s disease. They note that the side effects to be expected from inhibitors of ?-secretase would mainly affect early development, while inhibition of gamma-secretase causes problems in the adult organism. Yueming Li, who studies gamma-secretase at the Memorial Sloan-Kettering Cancer Center in New York still thinks that both secretases remain appealing drug targets. ’It may nevertheless prove possible to find a therapeutic window in which partial inhibition of BACE1 and/or ?-secretase activity could reduce A? production without significant obliteration of their activity for other substrates,’ Li told Chemistry World.
In a separate study, Mathias Jucker’s team at the University of T?bingen, Germany, and Lary Walker at Emory University in Atlanta, Georgia, US, have investigated the ’seeding’ of amyloid deposits that occurs when amyloid-containing extracts are injected into the brains of a mouse model of Alzheimer’s disease2.
’This crystallization-like mechanism bears important similarities to that of prion disease,’ said Walker, ’and adds to the evidence that a variety of protein-folding disorders may be initiated by the pathological seeding of certain proteins.’
While there is no evidence that prion-like ’infection’ plays a role in the natural course of Alzheimer’s disease, the findings could ultimately help to find a cure. Jes?s Zurdo, the executive director of UK biotech firm Zyentia, Cambridge, commented: ’This work opens the way to new animal models to study proteindepositional disorders that could accelerate the testing of new treatments for these conditions.’
Michael Gross
![]() Comment at the Chemistry World blog
Comment at the Chemistry World blog
Read other posts and join in the discussion
References
et al.Scienceet alScience313, 1781





No comments yet