Carbenes stabilise an electron-rich borane centre to create a potential new class of ligands for transition metal catalysts
Chemists in the US and Germany have achieved the remarkable feat of transforming a borane, an archetypal electron-accepting Lewis acid, into an electron-donating Lewis base. The researchers suggest that organic compounds with an electron-rich boron centre could form the basis of a new family of ligands for organometallic catalysts.
The research team, from University of California Riverside and Phillips-Universitat Marburg, used cyclic carbenes - in which a carbon has an unshared pair of valence electrons - to generate and stabilise an electron-rich boron centre.
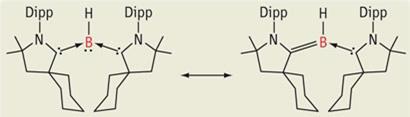
Boron has three outer electrons available for reaction, enabling it to combine with three single-electron donors to create a borane. This leaves a vacancy for two electrons to fill the outer orbital, making borane a strong electron acceptor - a Lewis acid.
’What we do is take boron with only one substituent, which gives it one electron,’ says Riverside’s Guy Bertrand. This monovalent compound is a borylene. ’So now there are four electrons, with four missing. Instead of using substituents that donate only one electron, we use two substituents that each give two electrons. We now have eight electrons, but not all of them are being used to make bonds - there is a lone pair available for reacting.’
The two-electron substituents are cyclic(alkyl)(amino)carbenes. Ordinarily the borylene would be highly unstable. However, carbenes not only have a spare pair of electrons, they also have a vacant orbital. This means that while the carbenes are donating electrons, they are also able to withdraw some of the electron density, providing sufficient balance to stabilise the electron-rich borane centre.
’What we have done is to transform a family of compounds which were acids into bases,’ says Bertrand. ’They look like amines or phosphines, which are important ligands for organometallic catalysts. This gives us potentially an entirely new family of ligands for transition metals.’
AnnMarie O’Donoghue, who studies carbene chemistry at the University of Durham, UK, says that the group’s stabilisation of borylene with the cyclic alklyamine carbene ligands is ’a remarkable breakthrough’. O’Donoghue adds: ’Such stabilised borylenes have significant potential as electron-donor ligands for transition metals, which could lead to new transition metal-borylene chemistry. The challenge that remains is to increase the reactivity of the borylene towards electrophiles, such as transition metals, by reducing the steric bulk and pi-acceptor ability of the ligands.’
Simon Hadlington
References
R Kinjo 10.1126/science.1207573






No comments yet