Metal organic frameworks are not just gas storage materials - they could also be used to create better fuel cells
New crystalline compounds could yield better materials for fuel cell applications, according to Canadian scientists. The researchers made conducting metal organic frameworks (MOFs) and showed they could be used to create the membranes that separate the gases in a proton exchange membrane fuel cell.
Often dubbed molecular ’sponges’ for their ability to mop up extraordinary quantities of gas or liquid in their cavernous pores, MOFs are widely thought of as gas storage materials, with the most obvious applications being in safe hydrogen storage and carbon capture. But George Shimizu at the University of Calgary in Canada and his team applied the mops to try to solve a different problem - high temperature proton conduction.
A proton exchange membrane is the insulating material between the two electrodes in a fuel cell, separating hydrogen on one side from air on the other and allowing hydrogen ions, but not electrons, to pass through. A major challenge in fuel cell technology is creating proton conducting membranes that work at an efficient operating temperature for a fuel cell - above 100?C. The team’s new conducting MOF, a honeycomb-structured organosulfonate called β-PCMOF2, does exactly that.
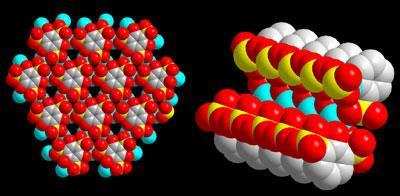
Oxygen atoms in the material’s sulfonate groups are exposed at the surface of its pores, creating a potential for proton conduction via hydrogen bond transport. The secret to high temperature conduction, however, is what is in the pores, says Shimizu. When loaded with water, β-PCMOF2 conducts at low temperatures, but using triazole molecules endows it with superior transport properties. ’We get proton conduction at 150 ?C,’ says Shimizu. ’Triazole is amphoteric and can accept and pass protons like water can. But it’s also less volatile than water and the right size to sit in those pores and prevent fuel crossover.’
G?rard F?rey, an expert on MOFs at the University of Versailles in France, says Shimizu’s work is scientifically of a high standard, but not a breakthrough for conducting MOFs. He also points out that the level of proton conduction needs to be vastly improved before the materials can be considered practical.
Shimizu admits the absolute level of proton conduction is currently at least an order of magnitude lower than it needs to be, but says the crystalline nature of MOFs provides plenty of scope for structural tuning, opening up the way to future generations of proton exchange materials.
According to F?rey, however, it is cost that will ultimately dictate whether MOFs are adopted by industry for fuel cell applications, and he thinks they are just too pricey. ’It’s only my opinion, but I don’t think it will give rise to applications soon, although it will remain academically interesting,’ he says.
Hayley Birch
References
et al, Nature Chem., 2009, DOI: 10.1038/NCHEM.402






No comments yet