For the first time, researchers have synthesised and characterised the ‘only possible structure’ with four hydroxyl groups linked to a carbon atom – methanetetrol.
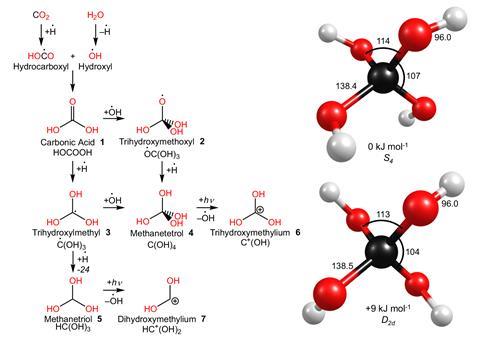
Also known as orthocarbonic acid, this molecule was first hypothesised in 1922 by physical chemist Ernst Wilke. Despite the stability and prevalence of tetravalent structures like orthocarbonic esters, the detection of the more modest methanetetrol – unstable and elusive – remained a mystery. ‘We used a different synthetic strategy,’ says lead author Ralf Kaiser from the University of Hawaii at Mānoa, US.
Similar to previous papers reporting the preparation of methanetriol, researchers resorted to interstellar conditions, simulated in the lab. To create this exotic environment, they exposed a mixture of carbon dioxide and water to highly energetic electron beams at ultra-high vacuum conditions and temperatures close to absolute zero. These conditions cause counterintuitive chemical reactions, such as the formation of otherwise unstable methanetetrol.
Under irradiation of the ‘interstellar ice’, researchers simultaneously synthesise and detect the unique tetra-alcohol. The ultra-high vacuum ‘avoids collisions with other molecules’ and prevents the otherwise usual decomposition to structures like water and carbonic acid.
To detect the presence of methanetetrol, the team combined computational and experimental techniques. The latter included photoionisation, which involves using powerful beams of UV light to split molecules into smaller ions, and mass spectrometry to ‘trap’ and find the different fragments. Although the identified fragments could come from contradictory combinations of atoms, isotopic substitution served as an easy solution – using marked carbon dioxide and deuterium oxide produced peaks specific to methanetetrol. ‘It confirmed the findings,’ says Kaiser.
After this ‘final frontier’, Kaiser is already planning the next extreme experiment – tetrahydroperoxymethane, a methane attached to four hydroperoxyl groups. ‘It would be a unique challenge,’ he adds.
References
J H Marks et al, Nat. Commun., 2025, 16, 6468 (DOI: 10.1038/s41467-025-61561-z)















No comments yet