
The first bulk synthesis of hexagonal diamond marks a milestone for carbon allotropes, offering researchers an opportunity to extensively characterise this unique material.
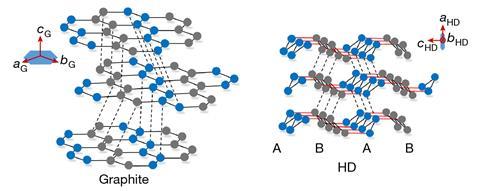
Diamond is one of the hardest materials known to exist in nature, arising from its structure in which carbon atoms covalently bond together in a perfect tetrahedral arrangement. Nearly 60 years ago, scientists predicted a harder, alternative form known as hexagonal diamond, which has a hexagonal lattice, rather than the cubic lattice adopted by conventional diamond.
Natural hexagonal diamond has been discovered on Earth and is thought to have formed during meteorite strikes when the immense temperatures and pressures can rapidly transform graphite into this rare form of diamond. However, only small grains of this natural hexagonal diamond have ever been discovered, mixed in with cubic diamond and graphite. Methods replicating the heat and pressure of a meteorite strike in the lab have often resulted in nanocrystalline structures of hexagonal diamond, with these samples often being impure, making it difficult to study hexagonal diamond in isolation.
‘Now we have made a millimetre-sized chunk of [near] pure hexagonal diamond,’ says Ho-Kwang Mao at the Centre for High Pressure Science and Technology Advanced Research in China.
To do this, Mao and his team in the US and China applied about 200,000 times atmospheric pressure to a single crystal of pure graphite using a diamond anvil cell. In situ x-ray diffraction before and after applying this pressure allowed the scientists to observe the microscopic conversion of graphite to hexagonal diamond. With the sample still under pressure, laser heating at 1400°C stabilised the phase, allowing the researchers to recover and subsequently study the near-pure sample.
‘This new two-step method provides the first definitive evidence of hexagonal diamond as a distinct and recoverable bulk material,’ says Eiichi Nakamura, an inorganic chemist at the University of Tokyo who has previously worked on carbon allotropes.
Through this method, the team synthesised crystals of near pure hexagonal diamond – containing a few microcrystals of the more familiar cubic diamond. The crystals ranged from 100μm to several millimetres in size, marking a first for forming a distinct and recoverable amount of the material.
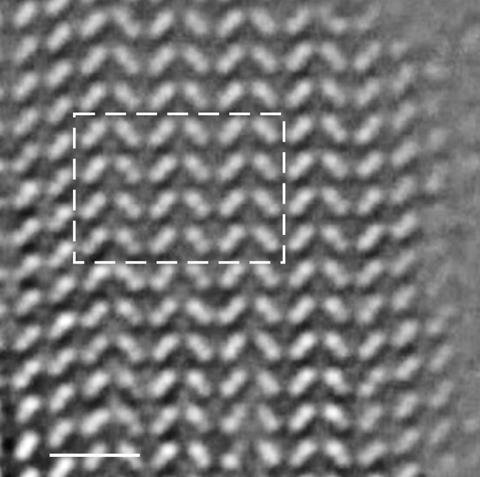
‘If you make [the bonding pattern of carbon atoms] three dimensional, there are only two ways of packing the layers,’ says Mao. ‘There’s ABC packing within cubic diamond, and AB packing for hexagonal.’ High-resolution transmission electron microscopy confirmed that the sample had AB stacking of buckled honeycomb layers, a structure indicative of hexagonal diamond.
The scientists probed the structure further using various spectroscopic techniques. ‘We found that one of the bonds between the layers is actually shorter, compared to the other three, so this helps explain why the structure is stronger [compared with cubic diamond],’ says Mao. Results also showed that all bonds were sp3 σ bonds, with no sp2 π bonds that would signal the presence of graphite.
The team tested the hardness of the material using a 1mm diameter disc of hexagonal diamond, finding that the hardness was comparable to natural diamond, due to minor cubic diamond defects. Future efforts will likely focus on refining synthesis conditions, with Nakamura noting that ‘this [synthetic] breakthrough marks a milestone in the study of carbon allotropes’.
References
L Yang et al, Nature, 2025, DOI: 10.1038/s41586-025-09343-x















No comments yet