Nitric oxide may have been used to treat angina in China since about 800 AD, says Anthony Butler.
Nitric oxide may have been used to treat angina in China since about 800 AD, says Anthony Butler.
Angina pectoris is a common heart condition among people, particularly men, over a certain age. It manifests itself as a severe pain beneath the breastbone that sometimes radiates into the left shoulder and down the inner side of the left arm. A feeling of suffocation may accompany the pain. Attacks are precipitated by a range of events: strenuous exercise, emotional reactions and even exposure to cold and wind.
The pain is caused by insufficient oxygen reaching the muscles of the heart because of narrowing of the cardiac arteries. This is due to a process similar to the furring of a water pipe. The fur, or atherosclerotic plaque, appears to prevent muscles lining the arteries from relaxing. Because of reduced blood flow through these arteries, waste products accumulate in heart muscle, leading to anginal pain.
An anginal attack abates if the sufferer rests but more rapid relief can come from one of a number of drugs, the most frequently used of which is glyceryl trinitrate (GTN), a component of dynamite and itself a high explosive.
Although its use in the treatment of angina was first described in 1879 by William Murrell in a report in The Lancet, the reason for GTN’s success was not understood until 1987, when nitric oxide (NO) was first suggested as the messenger molecule responsible for arterial muscle relaxation. When GTN is administered during an anginal attack, the nitro group is metabolised into NO, probably in the wall of the artery, which effects vasodilation: more blood flows and relief results.
GTN was first made by the Italian chemist Ascanio Sobrero around 1847 and, in view of its explosive nature, it is something of a miracle that he survived to tell the tale. Unless detonated GTN is relatively safe but, if it does experience a physical shock, the consequences are catastrophic. To moderate its action Alfred Nobel, the 19th century Swedish entrepreneur, incorporated it into kieselguhr, a porous material consisting largely of silica, and gave the world dynamite. It made him a personal fortune, not so much from its use in warfare, but from its widespread use in blasting. However, Nobel’s success with dynamite had its tragic side. His younger brother, Emil, and a number of other workers were killed in an explosion in 1863 during its development. By a curious twist of fate Nobel developed angina towards the end of his life and was treated with GTN, the very substance that had brought him so much wealth.
Safe at work?
During World War I women were employed in large numbers to pack GTN into munitions. Many complained of severe headaches and this was traced to the absorption of GTN through the skin, leading to an unhealthy lowering of blood pressure. Rather curiously the headaches disappeared by the end of the working week but returned on the following Monday. This we now know was due to the worker developing a tolerance to GTN, which was lost during the weekend. Thus it was common for Swedish munitions workers to place a little GTN in the band of the hat they wore at weekends to prevent ’Monday morning headaches’.
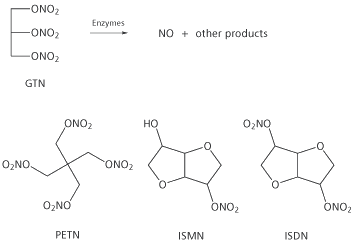
Other compounds related to GTN which are used therapeutically include pentaerythrityl tetranitrate (PETN), isosorbide mononitrate (ISMN) and isosorbide dinitrate (ISDN) but none is used as widely as GTN. Surprisingly, no tolerance effect is seen for PETN despite the similarity of its structure to GTN (see below).
GTN and similar vasodilators
Amyl nitrite has similar therapeutic effects to GTN but is too volatile for convenient medical use. During the 1960s amyl nitrite was used as a recreational drug and known as ’poppers’. Its use probably increased blood flow to the brain and caused a feeling of euphoria. In the early days of the Aids pandemic it was briefly thought that amyl nitrite might be the cause. Both amyl nitrite and GTN are substrates for the enzyme xanthine oxidoreductase, which converts them into NO. This could explain why they are effective in treating angina. However, very high concentrations of the two drugs are required to make the enzyme active and other routes or other enzymes, as yet unknown, may be more efficient.
The presence of groups containing both oxygen and nitrogen in GTN and in amyl nitrite makes it likely that both act as vasodilators by transformation into NO. Equally it suggests that other compounds containing groups with the same constituent elements could also be vasodilators. One such compound, and a particularly unlikely vasodilator, is sodium nitroprusside, Na2[Fe(CN)5NO], first reported by the Scottish chemist Lyon Playfair in 1849. When infused into the bloodstream a solution of sodium nitroprusside is highly effective in lowering blood pressure by dilating vessels and it is sometimes used during surgery. Why it is so effective is something of a mystery, but the presence of the NO ligand is suggestive of a pathway involving NO release. Nitroprusside is normally a particularly stable ion but NO release can be brought about by a photochemical process.
hv
[Fe(CN)5NO]2- __________> [Fe(CN)5H2O]2-+NO
It is possible that sodium nitroprusside acts as a vasodilator because of the intense lighting in the operating theatre. However, it is difficult to test this without killing the patient. Also, sodium nitroprusside acts as a vasodilator even in the absence of light, although less effectively, and so photochemical release of NO cannot be the full story.
The presence of five cyano ligands in nitroprusside has led to caution in its use, especially since reports of patient death by cyanide poisoning. In nitroprusside the cyano ligand is not labile but once aquapentacyanoferrate has been formed, as in the photochemical reaction, the cyano ligand can then exchange with a water molecule and so cyanide release may occur after (rather than during) the hypotensive process. The body can tolerate a certain amount of cyanide because of the presence of an enzyme, rhodanase, which converts it into harmless thiocynanate. However, it is highly unlikely that any regulatory body today would allow the use of sodium nitroprusside in medicine.
’NONOates’
In the 1960s Russell Drago, an American chemist, made and characterised a number of adducts of NO with secondary amines and coined the term NONOate for the products. They are now known as diazeniumdiolates. Larry Keefer and Joe Hrabie at the National Cancer Institute in Maryland have subsequently explored the properties of these compounds fully (Chem. Br., July 2000, p30).
Release of NO by diethyl NONOate

S-nitrosothiols deliver NO

Diethylamine NONOate releases NO in a clean, first-order reaction with a half-life of 2.1 minutes at 37?C and pH 7.4. Other NONOates decompose much more slowly and so these compounds provide an admirable source of NO with controlled delivery. One anxiety in their clinical use is the simultaneous release of a secondary amine. Because NO in an oxygen-rich environment can readily form a nitrosating species (probably N2O3), there is the slight danger of formation of a carcinogenic secondary nitrosamine. So far there has been no direct evidence of this hazard.
The use of S-nitrosothiols as a means of NO delivery has been much investigated in recent years. These compounds decompose cleanly with release of NO and formation of a disulphide.
2RSNO ___> RS-SR + 2NO
If the thiol from which the S-nitrosothiol is made is a naturally-occurring amino acid then all the components are devoid of hazard. Unfortunately S-nitrosocysteine is too unstable for use and most research has been carried out on S-nitroso-N-acetylpenicillamine (SNAP) but N-acetylpenicillamine and its disulphide are not naturally occurring. SNAP is frequently used in physiological experimentation as a source of NO but not medically. If the acetyl group of SNAP is replaced by one with a longer alkyl chain (as in SNVP) the compound’s lipid solubility is increased and it appears to become anchored in tissue it contacts. This results in localised and sustained release of NO. It is thought that S-nitrosothiols occur naturally in blood plasma (principally S-nitrosoglutathione) and may play some part in the normal metabolism of NO.
Age-old treatment
Is there any documentary evidence for the treatment of angina involving an NO-donor drug before the advent of modern medicine? Possibly. In 1901 an amazing collection of manuscripts was found in a walled-up cave in a Buddhist shrine in central Asia, called Dunhuang, by the British explorer Sir Aurel Stein. They were probably hidden there by Buddhist priests around 1000 AD as Islam swept into China. The collection includes domestic items, religious texts in many languages and some medical manuscripts. One manuscript describes what is obviously a treatment for the pain associated with angina. The patient is instructed to place nitre (potassium nitrate, KNO3) under the tongue and leave it there while carefully conserving the saliva. It guarantees a cure. (Nitre was well known to the Chinese because it is a component of gunpowder.) Under the tongue, there are colonies of bacteria, some of which contain the enzyme nitrate reductase, which converts nitrate into nitrite. Nitrite can be a source of NO because of the reaction.
2HNO2 ___> NO2 + NO + H2O
Thus NO is formed from nitrite under acid conditions. Tissue starved of oxygen, as in angina, is more acidic than healthy tissue and so, if nitrite is taken into the blood stream through the tongue, it could release NO in the oxygen-starved tissue of cardiac arteries and bring about vasodilation. It may be that the therapeutic use of NO in China predates that in the West by many centuries.
The discovery of widespread biological roles for NO over the past 16 years has brought about a minor revolution in our understanding of human physiology but, as yet, has not resulted in any new drugs. Compounds that release NO are easy to find; compounds that do so in specified tissues and nowhere else are much harder to source but the search goes on. The current world market in NO-related drugs is thought to be about $30,000m (?18,900m) and this is forecast to rise to $54,000m (?33,900m) by 2005. Cardiovascular disease is still one of the major causes of ill-health and death in the affluent West. It would be very surprising if new cardiovascular drugs based on the release of NO do not appear in the near future.
Source: Chemistry in Britain
Acknowledgements
Anthony Butler is Honorary Reader in Medical Science in the Bute Medical School, University of St Andrews.
Further Reading
- Life, death and nitric oxide, A. Butler and R. Nicholson. Cambridge: RSC, 2003.
- M. Feelisch and J. S. Stamler, ’Donors of nitrogen oxides’ in Methods in nitric oxide research, M. Feelisch and J. S. Stamler (eds). Chichester: John Wiley & Sons, 1996.






No comments yet