US scientists confirm the discovery of super-heavy element 114
US scientists have confirmed the discovery of element number 114, first made over a decade ago by a team in Russia. By smashing a high energy beam of calcium-48 ions into a plutonium-242 target, the US team managed to detect two nuclei of element 114, which is predicted by some to be bordering the so-called ’island of stability’ for superheavy atoms.
Yuri Oganessian and his team at Dubna, Russia, were the first to claim to have created nuclei of element 114 - but any such claim has to be thoroughly verified and the experiments repeated independently before the element can be considered for admission to the periodic table. Heino Nitsche and a team from the University of California at Berkeley spent eight days bombarding their plutonium target with calcium ions in an attempt to create the elusive element 114. ’We expected to see maybe six to eight nuclei, but we only found two!’ says Nitsche. He adds that the characteristic decay sequences and energies matched very well with the Russian data for two isotopes of element 114.

Like the Russians before them, Nitsche and his team used a high energy beam of 48Ca, which has eight extra neutrons compared to the most abundant isotope of calcium, 40Ca. When a 48Ca nucleus collides with a 242Pu nucleus, there is a small chance that the nuclei will fuse to create a compound nucleus with 114 protons (20 from calcium and 94 from plutonium) and 176 neutrons, explains Nitsche, ’but this is unstable and evaporates either three or four neutrons to make 287114 or 286114.’
This neutron evaporation is one of the reasons why it is difficult to confirm exactly what isotope is formed in a fusion experiment, says Nitsche, ’You know how they decay, but you can’t be 100 per cent sure if the number of neutrons that evaporates is correct because you’re not measuring the mass.’ The fact that some of the decay products can be unknown isotopes or even unknown elements adds to the difficulty, he adds.
Oganessian says he is very happy that Nitsche’s group have reproduced his team’s result.
Nitsche suggests that his confirmation of the data for 114 might be enough evidence for the International Union of Pure and Applied Chemistry (Iupac) to officially recognise the Russian group as its discoverers, which would allow them to propose a name for the element, but Oganessian is stoical about the process: ’Iupac will need to check the priority and accuracy of the discovery, and that’s a procedure that takes time - they have only just finished with element 112, and are still discussing the name and symbol.’ He adds that it will probably not be until the end of the year that they start discussing claims to the heavier elements, including 114 and 116.
Island life
Confirmation of the existence and lifetimes of isotopes of element 114 is a big step in the hunt for a tantalising ’island of stability’ predicted by theoreticians, where the lifetimes of super-heavy elements could be measured in hours, days or even millions of years. ’There are some calculations that say a nucleus with 114 protons and 184 neutrons would be stable,’ says Nitsche. The two isotopes confirmed by the Berkeley group, 286114 and 287114, have half-lives of 0.13s and 0.5s respectively, but the Russian data for a heavier isotope, 289114 - made by bombarding 244Pu with 48Ca, suggest a half-life of 2.6s. ’The half-lives increase as we get closer to 184 neutrons,’ says Nitsche, ’2.6 seconds is an absolute eternity - people are starting to plan chemistry experiments now, to see if 114 behaves like the elements above it in the periodic table.’
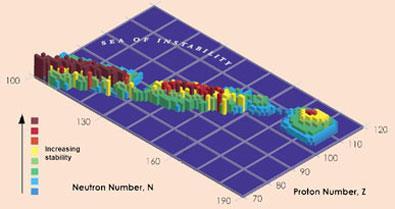
But Nitsche points out that they are still at least nine neutrons away from the predicted point of stability, and to make heavier isotopes is not easy. ’Some people say the island might not be until elements 120 or 126, or there might simply be a continuous increase in stability.’ He adds that experiments to make heavier elements are very important to aid theoreticians trying to understand the nucleus and make sensible predictions. Oganessian is also keen to find heavier elements, saying that his group has just begun a collaboration with scientists in the US to try and make element 117.
Phillip Broadwith
References
et alPhys. Rev. Lett.103, 132502 (DOI: 10.1103/PhysRevLett.103.132502)






No comments yet