UK chemists find a quick, inexpensive route to make a key sulfide reagent for asymmetric organic syntheses with the help of limonene
Chemists in the UK have discovered a quick, cheap and easy way to make a key sulfide reagent that can mediate the formation of chirally selective molecules needed for complex organic syntheses.
Chiral epoxides and aziridines are key intermediates in many organic syntheses. However, controlling their stereochemistry is tricky. It has been known for a long time that sulfide reagents can do this, but the reagents themselves either require multiple steps to synthesise, or else are relatively stereochemically unselective. It has been a longstanding problem to find a way to make selective reagents in a single step.
Now, Varinder Aggarwal’s team at the University of Bristol, UK, appear to have cracked the problem by reacting the terpene limonene - obtained from citrus fruit - with elemental sulfur in the presence of a second terpene. ’We include a magic ingredient in the reaction, gamma-terpinene, which reacts as a source of hydrogen,’ says Aggarwal. ’This makes the reaction go at a lower temperature and it becomes a much cleaner reaction.’
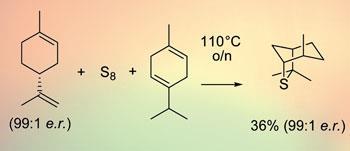
Depending on which stereoisomer of limonene is used, the result is a stereochemically pure isomer of the sulfur-containing compound isothiocineole. Various structures can be attached to the sulfide to create sulfonium ions and these can then be reacted with aldehydes or imines to produce the corresponding epoxide or aziridine in a chirally pure form depending on the chirality of the sulfide.
The Bristol team demonstrated that the resulting intermediates can be used in complex syntheses by carrying out a total synthesis of quinine.
’It has been major goal to find a sulfide reagent that can be made in one step and exhibit high selectivity,’ Aggarwal says. ’I think this could have a significant impact.’
Commenting on the work, Jon Parquette, an expert in organic synthesis at Ohio State University in the US, agrees: ’The fact that such remarkable enantioselectivities are achieved using a simple, readily available, chiral sulfide makes it likely that this method will receive a lot of attention in the future.’
Simon Hadlington
References
O Illa et al, J. Am. Chem. Soc., 2010, DOI: 10.1021/ja9100276






No comments yet