The industrially important synthesis of esters could be set to become greener and safer as German chemists have found a way to use carbon dioxide in place of carbon monoxide for alkoxycarbonylation. The new process does not need expensive or unstable reagents, and the catalyst is considerably cheaper than the palladium currently used.
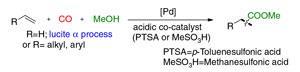
Carbonylation reactions involving the combination of alkenes with alcohols and carbon monoxide are widely used in industry to produce esters, which are turned into pharmaceuticals, polymers and detergents. Although carbon monoxide is highly toxic and flammable, it is still widely used by industry. It would be much safer and cheaper to use carbon dioxide in its place. The new process could also sequester small quantities of carbon dioxide from fossil fuel combustion, although the expense of extracting it from flue gases means that carbon dioxide is currently produced especially for industry.
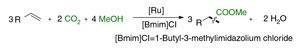
Matthias Beller and colleagues at the Leibniz Institute for Catalysis in Germany, found that triruthenium dodecacarbonyl can also catalyse the reaction. This metal complex is stable and significantly cheaper than palladium, which is currently used to catalyse industrial alkoxycarbonylation processes. The team investigated the reactions of a number of alkenes with a variety of alcohols using their catalyst achieved yields of over 90% in some cases.
Matt Clarke, whose group investigates catalysis at the University of St Andrews, UK, says the reaction is currently too inefficient for industry because, while the palladium catalysts used in the carbon monoxide process are tens of time more expensive, the concentrations needed are hundreds of times lower and the resulting reactions probably hundreds of times faster. The researchers acknowledge this and are working to optimise both the conditions and the catalyst. ‘I personally believe that by changing the conditions you can improve the system a little bit,’ says Matthias Beller, ‘but not to an extent that it will be industrially viable, so we need improved catalysts.’
Clarke agrees the work has potential. ‘With some further research it is entirely possible that the hurdles to making it competitive can be overcome,’ he says. ‘This is definitely something that should get supported because it would be important.’






No comments yet